Artigo
| Increased solubility and dissolution of gelucire® 50/13 solid dispersions containing carbamazepine |
|
Oscar G. da Silva NetoI,#; Rodrigo M. MartinsII,III,*,# I. Departamento de Engenharia de Materiais, Universidade Federal de Campina Grande, 58429-900 Campina Grande - PB, Brasil Received: 05/15/2025 *e-mail: rodrigo.martins@maisunifacisa.com.br Carbamazepine is widely used in the treatment of psychomotor epilepsy; however, its low aqueous solubility can limit therapeutic efficacy. In this study, solid dispersions were developed via hot melt extrusion to enhance the solubility of the drug and dissolution profile. Notably, the extrusion process induced a partial polymorphic conversion of carbamazepine from forms III to I, a less stable but more soluble form, which likely contributed to the improved performance. Dispersions were prepared using Gelucire® 50/13, polyethylene glycol (PEG) 4000, PEG 6000, and a PEG 4000/6000 blend at drug-to-carrier ratios of 1:9, 1:5, and 1:1. The formulation containing Gelucire® 50/13 at a 1:9 ratio exhibited the highest solubility (approximately three times greater than that of the pure drug) and a significantly enhanced dissolution profile. Characterization by X-ray diffraction, infrared spectroscopy, hot-stage microscopy, and thermal analysis confirmed the absence of physicochemical interactions between the drug and the carriers. Among the carriers tested, Gelucire® 50/13 emerged as the most promising for improving carbamazepine solubility and dissolution, potentially enhancing therapeutic efficacy and minimizing adverse effects. INTRODUCTION Carbamazepine (CBZ) is among the first-line recommended medications for the treatment of seizures. However, side effects lead one-quarter of epilepsy patients to discontinue treatment. In epilepsy treatment, for instance, the maintenance dose of CBZ ranges from 800 to 1200 mg day-1 in divided doses, which increases the intensity of adverse effects due to the need for progressively higher dose adjustments.1 However, CBZ is considered a poorly soluble drug, making it a candidate for strategies that can enhance its solubility and, consequently, reduce the administered dose, thereby minimizing its adverse effects.2 Improvements in the solubility and dissolution properties of active pharmaceutical ingredients (APIs), like CBZ, have grown significantly in recent years due to the increasing demand for low-solubility drugs in the pharmaceutical market.3 Drugs with limited solubility present a challenge as they exhibit low bioavailability and dissolution rates. Numerous strategies can be applied to enhance API solubility.4 However, as a first approach, less complex and costly methods should be prioritized. One such method is the production of solid dispersions, which involve pharmaceutical formulations where the drug is dispersed in a biologically inert matrix, generally to increase oral bioavailability.5 As a result, in vitro release is enhanced compared to conventional pharmaceutical formulations. The types of matrices used in the preparation of solid dispersions include hydrophilic polymers such as polyethylene glycol (PEG), polyvinylpyrrolidone (PVP), and low molecular weight materials such as sugars or lipophilic excipients (waxes and lipids).6 Despite being extensively researched, the application of solid dispersions in pharmaceutical products remains limited. This limitation may be due to challenges in preparation methods, reproducibility concerning the physicochemical properties of pharmaceutical formulations, scale-up of production methods, and/or stability issues.7 Nevertheless, depending on the API, solid dispersions can be a cost-effective alternative for developing innovative pharmaceutical products that may exhibit reduced adverse effect intensity. Therefore, the present study aimed to prepare CBZ solid dispersions with the carriers poloxylglyceride (Gelucire® 50/13) and polyethylene glycols (PEG 4000 and PEG 6000) to investigate improvements in solubility and dissolution properties.
EXPERIMENTAL Preparation and characterization of solid dispersions Preparation of solid dispersions Gelucire® 50/13, PEG 4000, and PEG 6000 were used as carriers. Each polymer was heated to 10 ºC above its melting point using a heating plate. CBZ was then gradually added under gentle stirring in different proportions. The molten material was subsequently cooled for 60 min in a freezer (-18 ± 1 ºC). Each sample was pulverized using a mortar and pestle and then sieved to calibrate the particle size, ensuring that it did not exceed 300 µm (50-mesh sieve). Preparation of physical mixtures Physical mixtures of the solid dispersion produced with the polymer that provided the best solubility were prepared for comparison in physicochemical characterization assays. Gelucire® 50/13 was used to prepare the physical mixture in CBZ:carrier ratios of 1:9 (10% CBZ), 1:5 (16.7% CBZ), and 1:1 (50% CBZ). Each sample was manually homogenized in an appropriate container for 10 min, followed by sieving to calibrate the powder mixture, obtaining particles smaller than 300 µm. Solubility study of solid dispersions The solubility test was conducted at 37 ± 1 ºC in purified water under controlled magnetic stirring for 48 h in a temperature-controlled oven. The tested ratios were 1:9, 1:5, and 1:1 (CBZ:polymer). The evaluated polymers were PEG 4000, PEG 6000, a 1:1 PEG 4000/PEG 6000 mixture, and Gelucire® 50/13. The solid dispersions of these polymers were weighed and added to water at a concentration of 20 mg mL-1, followed by stirring under controlled temperature as described above. Subsequently, the samples containing PEG 4000 and PEG 6000 were filtered through a 0.45 µm Millipore membrane using a vacuum system. Solid dispersions composed of Gelucire® 50/13 were filtered using a 0.45 µm Millipore membrane with vacuum assistance, followed by a second filtration using 0.22 µm Millipore filters. These samples were diluted, and their absorbance was determined by high-performance liquid chromatography (HPLC). All analyses were performed in triplicate. In vitro dissolution studies The dissolution rates of CBZ and solid dispersions were determined using the paddle method (USP apparatus 2) at a stirring rate of 75 rpm and 37 ºC in a dissolution apparatus (NE 330-8, Nova Ética, Vargem Grande Paulista, Brazil). The dissolution medium was 900 mL of deionized water, and the samples contained 180 mg of solid dispersion (SD), corresponding to 18 mg of the drug. Aliquots (5 mL) were withdrawn at 0, 5, 10, 15, 30, and 60 min, filtered (0.22 µm), and their drug content was determined by HPLC at 340 nm. The samples were tested in sextuplicate. Quantitative determination of CBZ The quantitative determination of CBZ was performed using chromatographic analysis with a Shimadzu (Kyoto, Japan) liquid chromatography system equipped with an LC-10 AT VP solvent pump unit and an SPD-10AVP UV-visible detector operating at 340 nm. Samples were manually injected through a Rheodyne injector (20 µL loop). Separation was performed on a C18 Shim-pack CLC-ODS column (280 mm × 4.6 mm, 5 µm) with a pre-column C18 Shim-pack CLC-ODS (10 mm × 4.6 mm, 5 µm). The mobile phase consisted of acetonitrile:water (35:65, v/v) at a flow rate of 1 mL min-1. Data were collected using a Chromatopac C-R6A integrator (Shimadzu).8 The HPLC method employed for CBZ quantification was previously validated, considering parameters such as sensitivity, linearity (1-30 mg mL-1), accuracy (coefficient of variation of 2.45%), and precision (coefficient of variation of 3.67%). The calibration equation was y = 35926x + 9238.3, with a linear correlation coefficient of r = 0.9980. Physicochemical characterization Thermogravimetric analysis (TG) CBZ and Gelucire® 50/13 were subjected to thermogravimetric analysis using a DTG-60 (Shimadzu, Kyoto, Japan). A sample of 5.00 ± 0.01 mg of each material was placed in an alumina crucible and heated from 30 to 600 ºC at a rate of 10 ºC min-1 in a nitrogen atmosphere with a flow rate of 30 mL min-1. The obtained data were analyzed using TA60 software (Shimadzu). Differential scanning calorimetry (DSC) Physical mixtures and solid dispersions of CBZ/Gelucire® 50/13 at 1:9, 1:5, and 1:1 ratio were analyzed using differential scanning calorimetry (DSC) with a DSC 50 Shimadzu device. An amount of 4.00 ± 0.05 mg of each sample was weighed and sealed in an aluminum sample holder. The samples were heated from 30 to 300 ºC at a rate of 10 ºC min-1 under a nitrogen atmosphere flowing at 50 mL min-1. The obtained data were analyzed using TA60 software (Shimadzu). Hot-stage microscopy (HSM) study Physical mixture (1:9) and solid dispersion (1:9) samples of CBZ in Gelucire® 50/13 were analyzed using hot-stage microscopy (HSM). A Zeiss Axio Cam HRC microscope equipped with a Linkam TMS 93/THSG 600 heating plate system was used. A small amount of each sample was placed in a sample holder and heated from 30 to 200 ºC at a rate of 10ºC min-1. X-Ray powder diffraction study Physical mixture (1:9), solid dispersion (1:9), CBZ, and Gelucire® 50/13 samples were subjected to X-ray diffraction analysis. The equipment used was a D5005 Bruker-Siemens X-ray diffractometer, equipped with a sealed copper source (λ = 1.5418 Å), operating in the 2θ range of 2 to 50º at an angular speed of 0.02º s-1, with a voltage of 40 kV and a current of 30 mA. Infrared spectroscopy (IR) study Physical mixture (1:9), solid dispersion (1:9), CBZ, and Gelucire® 50/13 samples were analyzed using infrared spectroscopy with an FTIR Nicolet Protege 450 device. The samples were mixed with potassium bromide (KBr) in a quartz mortar and pressed into mini-pellets to obtain translucent discs. Spectra were obtained in the wavenumber range of 4000-400 cm-1, and the characteristic peaks were analyzed.
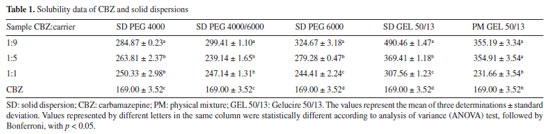
RESULTS AND DISCUSSION Water solubility tests The solubility of a drug is of utmost importance, as it directly influences the absorption rate of the active ingredient and the dose that should be administered. Poorly soluble drugs can become a limiting factor in dissolution. Therefore, strategies employed to increase the solubility of active ingredients are extremely important to ensure greater patient safety.9 Table 1 shows the solubility data of solid dispersions using the polymers evaluated in this study.
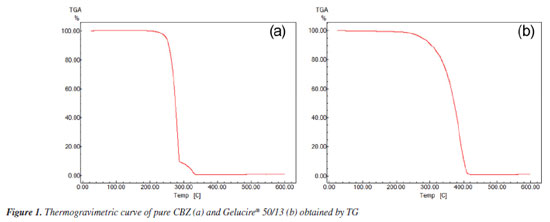
All solid dispersions studied were able to improve the solubility of CBZ. At a 1:9 ratio, the release of CBZ into its soluble form was the highest compared to the other ratios. Among these, the solid dispersion composed of Gelucire® 50/13 was the most effective, exhibiting 2.90 times higher solubility than pure CBZ, followed by 1.94 times when the carrier used was PEG 6000, 1.79 times for the 1:1 PEG 4000:PEG 6000 ratio, and 1.71 times for PEG 4000. These results indicate that Gelucire® 50/13 at this ratio provided better aqueous solubilization of CBZ than the other carriers. For comparison with the solid dispersion composed of Gelucire® 50/13, an assay was conducted using physical mixtures of CBZ and Gelucire® 50/13 at the same ratios (1:1, 1:5, and 1:9), where the results were similar for the 1:5 ratio. At the 1:9 ratio, the solid dispersion was more efficient than the physical mixture. One possible explanation for the superior performance of solid dispersions containing Gelucire® 50/13 compared to those prepared with polyethylene glycol (PEG) lies in the distinct physicochemical properties of these carriers. The Gelucire® carriers are an amphiphilic excipient composed of glycerides and polyethylene glycol esters of fatty acids, which enables it to form micelles in aqueous media, enhancing the apparent solubility of poorly water-soluble drugs through micellar solubilization. In addition, Gelucire® significantly improves the wetting of drug particles by reducing the interfacial tension, facilitating dispersion in the dissolution medium. Furthermore, its low melting point allows the formation of a uniform, porous matrix that improves water penetration and drug release.10 The solubilization of the drug can be influenced by specific types of chemical interactions that may depend on the structure of both the drug and the polymer. These specific interactions can easily occur with mono-, di-, and triglycerides, fatty alcohols, and polyglycosylated fatty acid esters, leading to an increase in solubility.11 Regarding the performance of solid dispersions at a 1:9 ratio (10% active ingredient), their efficiency is already well-documented in the literature and can be explained by several factors, such as the reduction in drug crystallinity due to an increase in the contact surface between the drug and the dispersing medium, consequently leading to greater dispersion of the drug in this medium. The drug particles may also be in a molecular form, or the manufacturing process itself may have promoted the formation of lower-melting-point polymorphs or an amorphous state in the solid dispersion.11 Techniques such as DSC, X-ray diffraction, electron microscopy, among others, are used for physicochemical characterization and can lead to a better understanding of possible drug-polymer interactions. Based on the solubility study results, the solid dispersion of CBZ in Gelucire® 50/13 at a 1:9 ratio was selected for further investigation. Initially, the CBZ:Gelucire® 50/13 solid dispersion samples were characterized through thermal analysis, powder X-ray diffraction, hot-stage microscopy (HSM), and infrared spectroscopy. Thermoanalytical studies Thermogravimetric analysis (TG) Thermogravimetric analysis was performed on CBZ and the polymeric carrier to determine the maximum temperatures at which these compounds remain stable, considering that during the melting process for the production of solid dispersions, the materials are subjected to thermal heating for a certain period. Figure 1a presents the thermogravimetric behavior of CBZ, showing the percentage of mass loss (%) during heating from 30 to 600 ºC at a constant rate of 10 ºC min-1. CBZ exhibited two decomposition stages. The first stage began at 252.91 ºC and ended at 286.59 ºC, with a mass loss of 90.29%. The second decomposition stage extended from 286.59 to 333.74 ºC, resulting in an additional mass loss of approximately 9%, leaving a final residual mass of 0.71%.
Figure 1b shows the thermogravimetric behavior of Gelucire® 50/13, with the percentage of mass loss under the same conditions applied to CBZ. Gelucire® 50/13 exhibited a single decomposition stage, starting at 324.12 ºC and ending at 408.43 ºC, with a mass loss of 98% and a final residual mass of 2%. In conclusion, the thermogravimetric analysis clearly indicates that both CBZ and Gelucire® 50/13 are stable at temperatures below 200 ºC, undergoing no decomposition. Therefore, the solid dispersion preparation process does not alter the chemical composition of these constituents. DSC analysis The DSC technique was employed to detect possible interactions between CBZ and Gelucire® 50/13 by analyzing their physical mixtures and to determine if the solid dispersion preparation process led to any changes in the thermal profile of their components. Figure 2 shows the calorimetric profile of Gelucire® 50/13. The calorimetric analysis of this polymer revealed a single endothermic peak at 45.49 ºC with a broad melting range, characteristic of such compounds. In contrast, CBZ exhibited two initial peaks indicative of an endothermic event. The first, a small peak, corresponds to the fusion of the β form or form III at 167 ºC, followed by a second peak corresponding to the fusion of the α form or form I at 192 ºC. A third event, an exothermic peak, occurs around 230 ºC.12
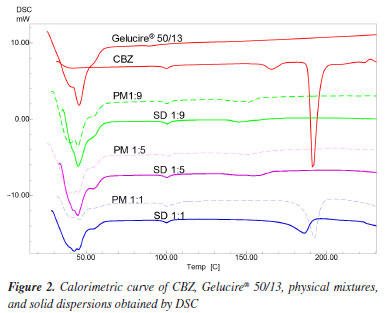
CBZ shows enantiotropic polymorphism, meaning that there is a transition temperature below the melting points of both polymorphs, at which they have the same free energy and can convert between each other. Above this transition temperature, the form I, which has the higher melting point, has the lowest free energy and is more stable. Below this transition temperature, however, the form III, which has the lower melting point, has the lowest free energy and is therefore more stable. The transition temperature of the enantiotropic form of CBZ is around 71 ºC. Therefore, at room temperature, form III is the most stable form. Consequently, the endothermic peak observed at 167 ºC in the experiment indicates that the CBZ analyzed is in form III,13 and the peak corresponding to form I occurred due to the DSC analysis process itself. This result is supported by X-ray diffraction data of the powders. The calorimetric profile of the solid dispersions and physical mixtures of CBZ and Gelucire® 50/13 in different proportions (1:9, 1:5, and 1:1) and their comparison with the CBZ and polymer profiles are shown in Figure 2. The PM (physical mixture) and SD (solid dispersion) samples with a 1:1 ratio showed both the polymer and drug fusion peaks. Due to the reduced amount of CBZ in the 1:5 and 1:9 ratios, the CBZ fusion peak was not detected, and only the polymer fusion peak was observed (Table 2).
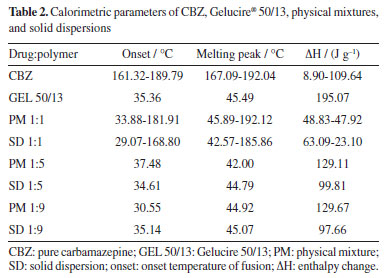
All the solid dispersions of CBZ showed lower enthalpy values than their respective physical mixtures (Table 2) when compared to each other. A plausible explanation for these values is that CBZ, when dispersed in the carrier, dissolves more quickly in it than in the physical mixtures during the DSC analysis process, leading to a decrease in the enthalpy values.14 The absence of the carbamazepine melting peak in the DSC thermograms of low drug-loading solid dispersions suggests either amorphization or molecular dispersion of the drug within the carrier matrix, or the presence of drug crystals below the detection limit of the DSC. However, it cannot be conclusively stated that the CBZ is entirely in the amorphous state due to the absence of the endothermic peak of CBZ in the solid dispersions in the 1:5 and 1:9 ratios. Since the fusion of Gelucire® 50/13 occurs before the fusion of CBZ, it is possible that CBZ crystals dissolve into the molten polymer during the DSC analysis, preventing the device from detecting the endothermic peak of the drug fusion. This hypothesis is supported by the fact that the physical mixture, in the same proportions, also did not present the endothermic peak of CBZ, only showing the polymer fusion peak (Figure 2). In contrast, the 1:1 physical mixture and 1:1 solid dispersion showed the presence of crystalline drug, likely because the amount of carrier was not sufficient to solubilize all the CBZ crystals. Through the calorimetric study by DSC, it was found that there were no interactions between the drug and the polymer in the physical mixtures and solid dispersions studied. However, it was not possible to verify any crystalline changes in CBZ using DSC, which justifies the use of other techniques such as X-ray diffraction and hot-stage microscopy. Powder X-ray diffraction study Powder X-ray diffraction (PXRD) is a powerful technique for identifying crystalline solid phases. Each crystalline solid phase has a unique powder X-ray diffraction pattern, which serves as a basis for its identification.15 To investigate the solid-state of the drug and determine whether there were any changes in its crystalline morphology corresponding to polymorphic transitions, PXRD analyses were conducted. Figure 3 presents the diffractogram of CBZ. This was compared to the standard data from the database of the instrument (D5005 Siemens), where peak overlapping confirmed that the analyzed drug corresponds to CBZ in its polymorphic form III. The most intense peaks characteristic of this form were identified at 2θ angles of 13.1, 15.4, 16, 25, 27.5, and 32.1º.16
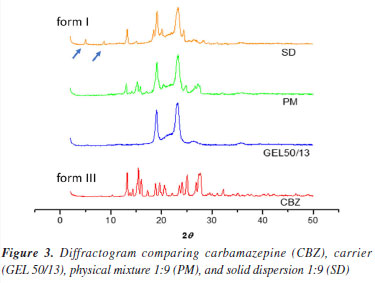
In Figure 3, the diffractogram of the carrier (Gelucire® 50/13) shows typical triglyceride peaks observed at 19.1 and 23.2º (2θ), known as short spacing.17 In the diffractogram of the 1:9 physical mixture, it is evident that no new peaks appeared that could be attributed to any of the polymorphic forms of CBZ. The peaks correspond to the original drug (form III), with the two most intense peaks being those of the carrier. In the diffractogram of the 1:9 solid dispersion, new peaks emerged at 2θ angles of 5.0 and 8.9º. These peaks characterize the polymorph I or α, which is likely formed during the preparation of the solid dispersion when the drug is subjected to heating (70 ºC) for a certain period. Therefore, the solid dispersion is probably composed of a mixture of polymorphs III and I.18 According to the literature,19 CBZ polymorph I can be obtained by heating form III. Thus, the production process of CBZ solid dispersion possibly promotes the transformation of a portion of form III into form I due to the heat supplied during the polymer melting process. This phenomenon is extremely important concerning the stability of the solid dispersion. Under storage conditions, form I tends to convert back to form III due to the enantiotropic phenomenon.20 These two forms differ significantly due to variations in their free energies,21 which lead to differences in their physicochemical properties, including solubility, potentially affecting the in vitro release profile. For instance, it was demonstrated that the dissolution rate of form I was higher than that of form III.22 Due to this factor, selecting appropriate storage conditions for solid dispersions is essential to ensure their long-term stability. Hot-stage microscopy (HSM) study Hot-stage microscopy (HSM) is an analytical technique that combines the best properties of microscopy and thermal analysis to characterize the physical properties of materials as a function of temperature.23 HSM can be used for the characterization of the solid-state of drugs, evaluation of crystalline forms and hydrates, and other physicochemical properties. The solid dispersion of CBZ in Gelucire® 50/13 at a 1:9 ratio and its corresponding physical mixture were analyzed by HSM, as shown in the photomicrographs presented in Figures 4 and 5.
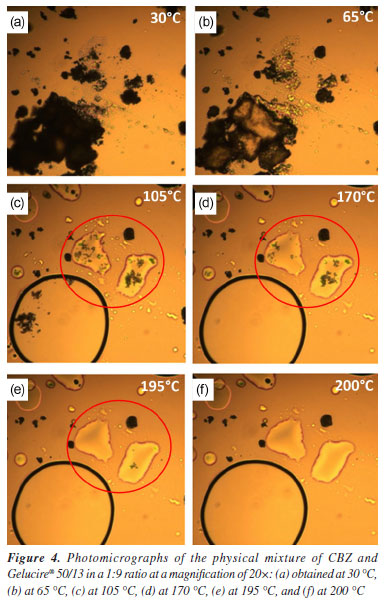
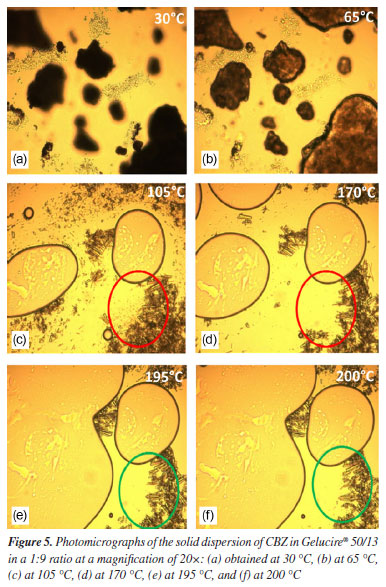
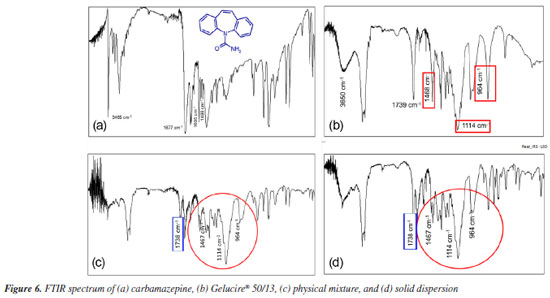
The onset of polymer melting in the physical mixture was observed at approximately 65 ºC (Figure 4b). In Figure 4c, the polymer is completely melted (105 ºC), and CBZ crystals (highlighted in the red-circled region) can be seen in their monoclinic form, characteristic of polymorphic form III.19 These findings are consistent with the results obtained from X-ray diffraction analysis. In Figure 4d, the fusion of CBZ crystals (red-circled region) was observed at approximately 170 ºC, which aligns with the DSC results, where the melting of this polymorph was detected at 167 ºC in the pure drug analysis. At 195 ºC (Figure 4e), the CBZ melting process is nearly complete, and at 200 ºC, the crystals are no longer visible. Figure 5 presents the photomicrographs of the CBZ solid dispersion in Gelucire® 50/13 (1:9). Similar to the physical mixture, the carrier melting began at 65 ºC (Figure 5b), and at 105 ºC, Gelucire® 50/13 was completely melted (Figure 5c), highlighting the presence of CBZ crystals. However, the majority of these crystals exhibited a trigonal shape, characteristic of CBZ polymorphic form I.19 This result was previously observed in the X-ray powder diffraction analysis, which confirmed the presence of polymorphic form I in the solid dispersion samples. At 170 ºC (Figure 5d), the fusion of CBZ crystals in form III was observed. This phenomenon becomes evident when comparing the red-circled region in Figure 5d with that in Figure 5c. Finally, the fusion of the trigonal crystals of form I was observed at 195 ºC (Figure 5e). In Figure 5f, the green-circled region shows a decrease in these crystals due to the melting process. The HSM analyses of the physical mixture and solid dispersion were of fundamental importance as they reinforced the X-ray diffraction results, which demonstrated the presence of crystalline forms in the samples. HSM also confirmed that the phenomenon observed in the DSC analysis - regarding the absence of CBZ endothermic melting peaks - occurred due to the partial dissolution of the drug crystals in the melted polymer, preventing the detection of their melting through the DSC technique. Infrared spectroscopy (IR) study Infrared (IR) spectroscopy was employed to investigate possible interactions between the drug and the polymer by analyzing their physical mixture and solid dispersion (both in a 1:9 ratio) in comparison with CBZ and Gelucire® 50/13. Figure 6a shows the FTIR spectrum of CBZ. The spectrum exhibited an intense peak at 3465 cm-1 corresponding to the stretching vibration of the -NH group, a peak at 1677 cm-1 related to the stretching of the carbonyl (C=O) group, which undergoes conjugation effects from the amide group, shifting its base value from 1715 to 1677 cm-1, a stretching at 1488 cm-1 due to the NH2 vibration, a stretching at 1605 cm-1 corresponding to the C=C vibration, and a vibration at 763 cm-1 characteristic of aromatic structures. These results are consistent with previous studies.24
Figure 6b presents the FTIR spectrum of Gelucire® 50/13. Gelucire® 50/13 displayed a broad band between 3650 and 3100 cm-1 due to the stretching of free OH groups, an intense peak at 1739 cm-1 belonging to the C=O group, as well as singlets at 1114 and 1468 cm-1 and a doublet at 964 cm-1, characteristic of polyethylene glycol groups.25 The spectra of the physical mixture and solid dispersion (Figures 6c and 6d, respectively) showed the presence of the same stretching and deformation bands related to CBZ and Gelucire® 50/13. This suggests that no interactions occurred between the drug and polymer functional groups. A potential interaction could involve the hydrogen of the CBZ amide group and the oxygen of the polyglycol chain, inducing a shift in the N-H vibration depending on the degree of interaction. This interaction would also lead to modifications in the C=O group of the polymer, which were not observed. Therefore, neither the solid dispersion production process nor the physical mixture induced modifications in the mentioned peaks. In vitro dissolution study The dissolution profile defines the dissolution rate characteristics of a drug in a solid pharmaceutical form. Dissolution studies are conducted to detect manufacturing deviations in the pharmaceutical formulation, ensure uniformity during batch production, and guarantee batch-to-batch reproducibility. Additionally, they assist in evaluating changes after product registration, aid in decision-making regarding bioequivalence studies, and contribute to establishing in vitro-in vivo correlation.26 Figure 7 shows the dissolution profile of the solid dispersion with CBZ. The dissolution rate of pure CBZ was approximately 25% in 15 min and reached only 45% release in 60 min. In contrast, the solid dispersion exhibited a superior release profile from the beginning of the dissolution process, reaching approximately 85% in 60 min. This increase in the dissolution profile of the solid dispersion compared to pure CBZ is possibly explained by an intensified solubilization effect provided by the carrier, which accelerates the solubilization process by increasing the wettability of the drug.
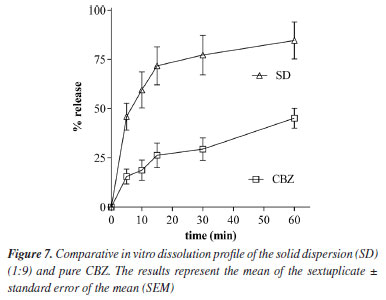
The results obtained showed that it was possible to significantly increase the dissolution rate of CBZ using Gelucire® 50/13 as a carrier. The available evidence suggests that the in vivo bioavailability of CBZ is not dramatically different between forms I and III, despite measurable differences in their dissolution rates in vitro. Nonetheless, maintaining CBZ in a stable and predictable solid form I is advantageous from a manufacturing and regulatory perspective, as it minimizes batch-to-batch variability, avoids conversion to less desirable forms during storage, and ensures long-term product stability. Moreover, formulations containing form I can be engineered (e.g., via solid dispersions) to improve wetting and apparent solubility, offsetting its intrinsically slower dissolution.27
CONCLUSIONS The present study demonstrated that solid dispersions of carbamazepine prepared with Gelucire® 50/13 significantly improved the solubility and dissolution rate of the drug compared to dispersions prepared with polyethylene glycol (PEG). The superior performance of Gelucire® 50/13 can be attributed to its amphiphilic nature, which promotes micellar solubilization, enhances drug wetting, and facilitates partial amorphization, factors that contribute to increasing drug availability in aqueous media. Moreover, the fusion method used to prepare the dispersions favored the transformation of CBZ from the metastable form III to the more stable form I, and the presence of Gelucire® may have contributed to maintaining form I during storage and dissolution. These findings reinforce the potential of Gelucire®-based solid dispersions as an effective and simple strategy to enhance the biopharmaceutical performance of poorly water-soluble drugs such as CBZ. Future studies may explore the long-term stability and in vivo performance of these formulations to confirm their clinical applicability.
DATA AVAILABILITY STATEMENT All data are available in the text.
AUTHOR CONTRIBUTIONS Oscar G. da Silva Neto was responsible for writing (original draft, review and editing), visualization, validation, methodology, investigation, formal analysis, conceptualization; Rodrigo M. Martins for writing (original draft, review and editing), visualization, validation, methodology, investigation, formal analysis, conceptualization; Denilson L. Rocha for methodology, investigation, formal analysis, conceptualization; Gustavo L. F. Barbosa for methodology, investigation, formal analysis, conceptualization; Maria E. C. B. Rangel for methodology, investigation, formal analysis, conceptualization; Wladymyr J. B. de Sousa for writing (review and editing), visualization, validation, methodology, investigation, formal analysis, conceptualization; Ana R. M. de Lima for validation, methodology, formal analysis; Luis A. P. de Freitas for writing (review and editing), visualization, project administration, fundraising.
REFERENCES
1. Sotoudegan, F.; Amini, M.; Sharifzadeh, M.; Samadi, N.; Sotoudegan, F.; AAPS Open 2024, 10, 12. [Crossref] 2. Kalantri, S. S.; Yadav, M. D.; Cryst. Res. Technol. 2024, 59, 2300296. [Crossref] 3. Munnangi, S. R.; Youssef, A. A. A.; Narala, N.; Lakkala, P.; Narala, S.; Vemula, S. K.; Repka, M.; Pharm. Res. 2023, 40, 1519. [Crossref] 4. Kumari, L.; Choudhari, Y.; Patel, P.; Gupta, G.; Singh, D.; Rosenholm, J.; Bansal, K. K.; Kurmi, B.; Life 2023, 13, 1099. [Crossref] 5. Bertoni, S.; Albertini, B.; Passerini, N.; Int. J. Pharm. 2025, 669, 125030. [Crossref] 6. Patel, K.; Shah, S.; Patel, J.; Daru, J. Pharm. Sci. 2022, 30, 165. [Crossref] 7. van der Merwe, J.; Steenekamp, J.; Steyn, D.; Hamman, J.; Pharmaceutics 2020, 12, 393. [Crossref] 8. Martins, R. M.; Siqueira, S.; Freitas, L. A. P.; Drying Technol. 2012, 30, 935. [Crossref] 9. Raines, K.; Agarwal, P.; Augustijns, P.; Alayoubi, A.; Brandl, A. B.; Brandl, M.; Chatterjee, P.; Chen, H.; Yu, Y. C.; Coutant, C.; Coutinho, A. L.; Curram, D.; Dressman, J.; Ericksen, B.; Falade, L.; Gao, Y.; Gao, Z.; Ghosh, D.; Ghosh, T.; Govada, A.; Gray, E.; Guo, R.; Hammell, D.; Hermans, A.; Jaini, R.; Li, H.; Mandula, H.; Men, S.; Milsmann, J.; Moldthan, H.; Moody, R.; Moseson, D. E.; Müllertz, A.; Patel, R.; Paudel, K.; Reppas, C.; Savkur, R.; Schaefer, K.; Serajuddin, A.; Taylor, L. S.; Valapil, R.; Wei, K.; Weitschies, W.; Yamashita, S.; Polli, J. E.; AAPS J. 2023, 25, 103. [Crossref] 10. Alshawwa, S. Z.; El-Masry, T. A.; Elekkhnawy, E.; Alotaibi, H. F.; Sallam, A.; Abdelkader, D. H.; Pharmaceuticals 2023, 16, 258. [Crossref] 11. Askarizadeh, M.; Esfandiari, N.; Honarvar, B.; Sajadian, S. A.; Azdarpour, A.; ChemBioEng Rev. 2023, 10, 1006. [Crossref] 12. Zhong, Z.; Yang, X.; Wang, B.; Yao, Y.; Guo, B.; Yu, L.; Huang, Y.; Xu, J.; CrystEngComm 2019, 21, 2164. [Crossref] 13. Nair, R.; Gonen, S.; Stephen, W. H.; Int. J. Pharm. 2002, 240, 11. [Crossref] 14. Bertoni, S.; Albertini, B.; Passerini, N.; Int. J. Pharm. 2023, 632, 122576. [Crossref] 15. Ali, A.; Chiang, Y. W.; Santos, R. M.; Miner.Eng. 2022, 12, 205. [Crossref] 16. Otsuka, M.; Ohfusa, T.; Matsuda, Y.; Colloids Surf., B 2000, 17, 145. [Crossref] 17. Passerini, P.; Perissutti, B.; Moneghini, M.; Voinovich, D.; Albertini, B.; Cavallari, C.; Rodriguez, L.; J. Pharm.Sci. 2002, 91, 699. [Crossref] 18. Sá Filho, A.; Martins, J. L. R.; Costa, R. F.; Pedrino, G. R.; Duarte, V. S.; Silva, O. N.; Napolitano, H. B.; Fajemiroye, J. O.; Int. J. Mol. Sci. 2024, 25, 9835. [Crossref] 19. Rustichelli, C.; Gamberini, G.; Ferioli, V.; Gamberini, M. C.; Ficarra, R.; Tommasini, S.; J. Pharm. Biomed. Anal. 2000, 23, 41. [Crossref] 20. Herboth, R.; Lyubartsev, A. P.; ACS Omega 2024, 9, 36718. [Crossref] 21. Kobayashi, Y.; Ito, S.; Itai, S.; Yamamoto, K.; Int. J. Pharm. 2000, 193, 137. [Crossref] 22. Kaneniwa, N.; Yamaguchi, T.; Watari, N.; Otsuka, M.; Yakugaku Zasshi 1984, 104, 184. [Crossref] 23. Lupone, F.; Padovano, E.; Casamento, F.; Badini, C.; Materials 2021, 15, 183. [Crossref] 24. Pinto, M. A. L.; Ambrozini, B.; Ferreira, A. P. G.; Cavalheiro, E. T. G.; Braz. J. Pharm. Sci. 2014, 50, 877. [Crossref] 25. El Hadri, M.; Achahbar, A.; El Khamkhami, J.; Khelifa, B.; Faivre, V.; Abbas, O.; Bresson, S.; Chem. Phys. Lipids 2016, 200, 11. [Crossref] 26. Rusdin, A.; Gazzali, A. M.; Thomas, N. A.; Megantara, S.; Aulifa, D. L.; Budiman, A.; Muchtaridi, M.; Polymers 2024, 16, 286. [Crossref] 27. Bugay, D. E.; Brush, R. C. In Remington: The Science and Practice of Pharmacy, 23rd ed.; Adeboyejo, A.; Green, C., eds.; Pharmaceutical Press: London, 2021, p. 313.
#Both authors are senior co-authors of this paper. |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access