Artigo
| Sustainable obtaining of antileishmanial compounds from Cedrela odorata L. demolition wood as an example of the circular bioeconomy |
|
Paulo Alan D. NogueiraI; Henrique C. dos SantosI; Claudete C. do NascimentoII; Bruno B. JensenIII; Antonia Maria R. FrancoIII; Pamela Emily T. IwabuchiIII; Maria da Paz LimaIV,* I. Programa de Pós-Graduação em Química, Universidade Federal do Amazonas, 69080-900 Manaus - AM, Brasil Received: 05/22/2025 *e-mail: mdapaz@inpa.gov.br Cedrela odorata L. (Meliaceae), popularly known as cedar, is a species that is valued for the quality of its wood, though its exploitation for decades has led it to be included on the list of Brazilian species of flora as an endangered species. The recycling of wood residues of this species generated by demolition is an excellent strategy for a circular economy. In this paper, we present the phytochemical study of C. odorata demolition wood for the sustainable production of antileishmanial compounds against promastigote forms of Leishmania (V.) guyanensis and Leishmania (L.) amazonensis. Classical chromatographic techniques were used in the purification of one sesquiterpene, eight triterpenes and five limonoids. The limonoid 7-deacetoxy-7a-hydroxy-gedunin was able to inhibit promastigote forms of L. guyanensis [IC50 (50% inhibitory concentration) of 26 μg mL-1] for 48 h, and gedunin showed moderate activity. The present study indicates that cedar demolition wood (Cedrela odorata), despite being about forty-five years old, still had its secondary metabolites, including those that are characteristic of the species such as limonoids, triterpenes of the tirucallane- and cycloartan-types. INTRODUCTION Cedrela odorata L. (Meliaceae), popularly known as cedar or red cedar, is a species that is valued for the quality of its wood. It has the Amazon as its center of origin, but its occurrence extends to other Brazilian biomes (Caatinga, Cerrado and Atlantic Forest).1,2 For decades, exploitation of its wood has represented a significant threat to this species, which led to its inclusion on the official list of Brazilian species of flora as an endangered species. Currently, C. odorata is considered a vulnerable species (VU).3 Some planting experiments of C. odorata have been conducted and have shown the species to be useful for the restoration of forests when planted for enrichment.4 Agroforestry systems using C. odorata associated with coffee have good growth in diameter and height and better resistance to attack by Hypsipyla grandella.5 Toona ciliata grafted on C. odorata has been used in Brazil to analyze possible chemical factors (secondary metabolites) that might be responsible for the attraction of H. grandella to C. odorata.6 Nogueira et al.7 present a review of the chemical compounds and biologic activities of the genus Cedrela that makes it clear that triterpenes, including limonoids, are predominant in various vegetative parts of Cedrela odorata. Limonoids are a secondary metabolite class of highly oxygenated modified triterpenes that are characteristic of the Meliaceae family. They are reported in C. odorata as an antifeedant, have antimalarial and anticancer activity and are Hsp90a inhibitors. The leishmanicidal activity was reported in limonoid-rich fractions from other species of Meliaceae, such as andiroba (Carapaguianensis Aubl.) oil used against Leishmania amazonensis8,9 and purified limonoids against L. major.10 However, there is a lack of studies on the use of purified limonoids against the protozoan species Leishmania (V.) guyanensis and Leishmania (L.) amazonensis. Leishmaniasis is endemic in the Amazon region, and the cutaneous form of leishmaniasis is the predominant form11 with few therapeutic options being available.12 Considering the vulnerability to extinction of the speciesCedrela odorata and the quality of its wood, the process of recycling residues generated by demolition is an excellent strategy for a circular economy that is not yet widespread, which indicates a gap to be explored. In this paper, we present the results of a phytochemical study on C. odorata demolition wood for the sustainable production compounds against the promastigote forms of Leishmania (V.) guyanensis and Leishmania (L.) amazonensis.
EXPERIMENTAL General experimental procedures Nuclear magnetic resonance (NMR) spectra were recorded on a Bruker Fourier 300 UltraShield (300 MHz for 1H and 75 MHz for 13C) and a Bruker Avance III 500 (500 MHz for 1H and 125 MHz for 13C), using deuterated solvents. Electrospray ionization mass spectra (ESI-MS) were obtained using a high-resolution mass spectrometer (MicroTOF-Q-II). Column chromatographic (CC) separations were performed on silica gel (70-230 and 200-300 mesh, Sigma-Aldrich) and Sephadex LH-20 (Sigma-Aldrich). Preparative thin-layer chromatography was performed with precoated silica gel plates (F-254, Merck). Acquisition of wood residues and identification The samples of demolition wood came from window frames that were used for about forty-five years in one of the buildings at the Instituto Nacional de Pesquisas da Amazônia (INPA, 3º05'44.1" S; 59º59'15.1" W) during its renovation (INPA document No. 608/2009). Species identification was confirmed based on its macroscopic anatomy, in addition to comparison with samples available in the xylotheque of INPA. Isolation of compounds The samples of C. odorata were extracted with hexane and then with methanol. The concentrated MeOH extract (8.6 g) was subjected to column chromatography (CC) over silica gel (70-230 mesh; h (height) × Ø (diameter) = 38 × 2.3 cm) and eluted with hexane, followed by hexane-EtOAc (2-30%) and EtOAc, which yielded twenty fractions (Frs.). Frs. 3 (70 mg), 8 (8.84 mg), 9 (101 mg), 11 (264 mg), 13 (354 mg), 17 (1.077 mg), 18 (205 mg) and 19 (1.284 mg) were subjected to new chromatographic fractionations. Fr. 3 was chromatographed in a silica gel column (70-230 mesh; h × Ø = 9 × 1.8 cm) eluted with hexane and then hexane-EtOAc (2-5%) to give compound 1 (25 mg). Fr. 8 was fractionated over silica gel CC (230-400 mesh; h × Ø = 42 × 1.8 cm) eluting with hexane and hexane-EtOAc (2-40%) to give 2 (4 mg). Fr. 9 was subjected to CC over silica gel (230-400 mesh, h × Ø = 44 × 2.8 cm), and eluted with CH2Cl2, which resulted in the purification of compounds 1 (37 mg), 2 (2.6 mg), 3a, b (mixture, 37 mg) and 4 (2.6 mg). Fr. 11 was chromatographed over cellulose, eluted with hexane and subfraction (subfr.) 2 was then purified using preparative thin-layer chromatography (silica gel; CH2Cl2) to give compound 5 (4 mg). Fr. 13 was subjected to preparative thin-layer chromatography, eluted with CH2Cl2-acetone (98:5) and resulted in the purification of 6 and 7 (2 mg) and 8 (15 mg). Fr. 17 was chromatographed over silica gel (70-230 mesh; h × Ø = 36 × 4.8 cm) eluted with CH2Cl2, CH2Cl2:MeOH (1-2%) and generated 23 subfractions. Fr. 18 (205 mg) was recrystallized with hot hexane and drops of acetone for purification of 9 (15 mg). Fr. 19 (1.284 mg) was subjected to column chromatography over silica gel (70-230 mesh, h × Ø = 36 × 4.8 cm), eluted with CH2Cl2, CH2Cl2:MeOH (1-2%) and gave compounds 10 (10 mg), 11 (13 mg), 12 (12 mg) and 13 (114 mg). Assay with Leishmania (V.) guyanensis and Leishmania (L.)amazonensis For this assay, we used promastigote forms of Leishmania (V.) guyanensis (strain MHOM/BR/1995/IM4147) and Leishmania (L.) amazonensis (strain MHO/BR/2009/IM5584), cryopreserved in the Laboratory of Leishmaniasis and Chagas Disease at INPA. The antileishmanial activity of the compounds was assessed by inhibiting the growth and mortality of promastigotes. The compounds were diluted in Roswell Park Memorial Institute (RPMI) culture medium with inactivated bovine fetal serum (iBFS) supplementation, filtered and then concentrations of 7.8 to 125 µg mL-1 were used for the assay. The positive control used was Pentacarinat®, and the negative control was dimethyl sulfoxide. For the bioassay, a 96-well plate with the test samples together with the parasites and the control was incubated in an oven at 25 ºC for 24 to 72 h. The bioassays were carried out in triplicate and the average number of live cells was used to calculate the 50% inhibitory concentration (IC50). The IC50 was calculated from the determination of antileishmanial activity, and the classification is based on a study by Osorio et al.13 for in vitro biological assays: highly active (IC50 < 10 μg mL-1), active (IC50, 10 to 50 μg mL-1), moderately active (IC50, 50 to 100 μg mL-1), and inactive (IC50 > 100 μg mL-1).
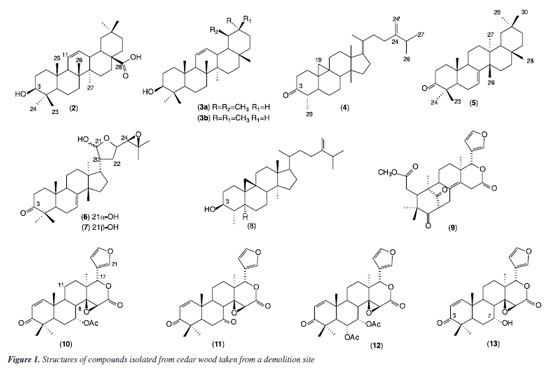
RESULTS AND DISCUSSION The phytochemical study of the methanolic extract of C. odorata demolition wood led to the isolation and identification of terpenes (sesquiterpene 1 and triterpenes 2-8), and limonoids (9-13), Figure 1. The 1H NMR spectrum of compound 1 showed characteristic hydrogen signals of the 1,2,4-trisubstituted aromatic system at δ 7.92 (d, 8.6 Hz), 7.35 (8.6, 1.6 Hz) and 7.91 (d, 1.6 Hz), assigned to ring A; ortho-coupled hydrogens of ring B at δ 7.28 and 7.21 (d, 7.6 Hz). An isopropyl group was verified at δ 3.71, two methyls linked to the aromatic ring at δ 2.63 and 2.54 and two methyls as a doublet at δ 1.36 (6.8 Hz). Thus, the bicyclic sesquiterpene was identified as cadalene (1), reported here for the first time in the species C. odorata. This sesquiterpene is normally found in some essential oils, but we obtained from the isolated form.
The identification of pentacyclic triterpenes 2, 3a, b and 5 was made based on NMR spectra. The 1H and 13C NMR spectra of 2 showed typical shifts of double bond of oleananes (δC 144.03 and 122.13) that, associated with the carbinolic signal at d77.67 and carbonyl at δ 177.99, allowed us to establish it as oleanolic acid previously reported in C. odorata stems.14 The 13C NMR of 3 was indicative of the mixture of the triterpene ursane (δC 124.4 and 139.5) and oleanane (δC 121.7 and 145.2), which were identified as α-amyrin (3a) and β-amyrin (3b). The 1H NMR spectra of 5 showed olefinic hydrogen signals at δ 5.36 (dd, 5.9 and 2.7 Hz), the 13C NMR spectrum showed carbonyl at δC 214.3 and olefinic carbon signals at δC 117.9 and 145.7, characteristic of a double bond between C-7 and C-8. The HMBC (heteronuclear multiple bond correlation) experiment showed the correlation of methyl at δH 1.08 (Me-26) with δC 145.7 (C-8), 43.1 (C-14) and 33.8 (C-15); the methyl at δH 0.89 (Me-27) correlated at δC 31.7 (C-12), 36.8 (C-13), 43.1 (C-14) and 48.9 (C-18). Based on the MS and NMR data, the structure of compound 5 was proved to be multiflorenone. This triterpene belongs to the migrated oleanane group whose analogues were identified by Cerdas-García-Rojas et al.15 and Ageta and Arai.16 They have restricted distribution in some botanical families, with only one report in Meliaceae (Sandoricum indicum).17 The tetracyclic triterpenes identified are cycloartane-type (4 and 8), and tirucallane with a side chain containing a hemiacetal and an oxirane ring (6 and 7). The 1H NMR spectrum of 4 showed a doublet at δH 0.63 and 0.41 (4.0 Hz) of a cyclopropane ring, and signals characteristic of a terminal double bond atdH 4.73 sl and 4.68 (d, 1.2 Hz). The 13C NMR spectra indicated a CO group (δC 213.5) and a terminal double bond (δC 156.8 and 105.9). The HMBC showed correlation of the cyclopropane hydrogens with δC 32.8 (C-1) and 47.1 (C-8), methyl hydrogen at δH 1.00 (Me-29) with δC 213.4. Thus, the analyses of the NMR spectra revealed that 6 and 7 are melianone epimers,18 identified from Amazonian species of Meliaceae,19 though not yet reported in C. odorata. Compound 4 was characterized as cycloeucalenone, reported in this paper for the first time in C. odorata. The 1H NMR spectrum of 8 showed signals similar to those observed for the triterpene 4, with a difference in the C-3 substitution due to the appearance of oxymethine hydrogen at δH 3.23 as ddd (10.7, 9.1, and 4.7 Hz), indicating the presence of β-configuration hydroxyl at position 3. The 13C NMR spectrum showed the displacement of the carbinolic carbon at δC 76.5, thus compound 8 was identified as cycloeucalenol, previously found in C. odorata L. grafted with Toona australis (F. Muell.) Harms.6,20 Identification of limonoids mexicanolide-type (9) and gedunin derivatives (10-13) was based on the NMR (1D), but the HSQC (heteronuclear single quantum correlation) and HMBC experiments were also used to complete or remove any unambiguous assignments of the compounds. 1H NMR spectrum of 9 indicated four methyls as singlets, a typical β-substituted furan and a carbomethoxy group (dH 3.68). The 13C NMR spectrum showed the presence of two six-membered ketones (δC 213.0 and 211.1) and a tetra substituted double bond (δC 133.3 and 125.3). The 1H and 13C NMR spectral data of 9 are similar to that of a mexicanolide previously isolated from C. odorata stem bark.21 The 1H and 13C NMR spectra of compounds 10-13 were indicative of gedunin-type limonoids.22 Thus, compound 11 was identified as gedunin, a known limonoid with reports in the heartwood of C. odorata23 and the stem of C. odorata grafted with T. ciliata.20 The spectral data of 11-13 were very similar to those of 10, except for the absence of an acetyl group at C-7, which was replaced by a keto group of 11 (7-deacetoxy-7-oxo-gedunin), the presence of an acetyl group at C-6 of 12 (6-α-acetoxy-gedunin) and the acetoxyl group in 7 was replaced by a hydroxyl of compound 13 (7-deacetoxy-7α-hydroxy-gedunin). The limonoid gedunin (10) and its derivatives 7-deacetoxi-7-oxo-gedunin (11) and 6α-acetoxi-gedunin (12) were subjected to an assay of their activity against promastigote forms of Leishmania spp. and their 50% inhibitory concentrations at different incubation times are presented in Table 1.
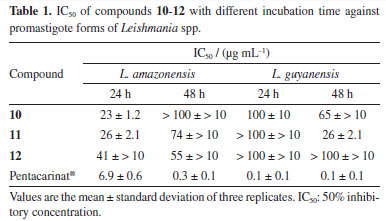
Limonoids or tetranortriterpenes are the main metabolites of the Meliaceae family and consist of highly oxygenated modified triterpenoids that exhibit a wide range of biological activities, including antileishmanial activity.23,24 In our assay of its antileishmanial activity, the limonoid deacetoxy-7-oxo-gedunin (11) was able to inhibit promastigote forms of L. guyanensis (IC50 = 26 μg mL-1) for 48 h, and gedunin (10) showed moderate activity. The limonoids test against L. amazonensis, 7-deacetoxy-7-oxo-gedunin (11) and 6-α-acetoxy-gedunin (12) showed moderate activity for 48 h (Table 1). The mechanism of action of limonoids against Leishmania spp. still needs further study, but an analysis of the structure-activity relationship of gedunin (10) and its derivatives (11 and 12) allows us to affirm the importance of the substituents at C-6 and C-7 of gedunin derivatives for the antileishmanial activity, making evident the importance of the carbonyl at position 7 of the molecule for activity against L. guyanensis. Nogueira et al.25 found moderate activity (IC50 of 39.4 μg mL-1) after 72 h in the test with the mexicanolide against L. guyanensis. Thus, comparing the ring systems of the mexicanolide-type limonoid with those of gedunin derivatives, it is suggested that the (D) δ -lactone ring and furan ring present in the two skeletons of the limonoids contribute to the activity against L. guyanensis. Almeida-Souza et al.8 observed that limonoid-rich fractions from andiroba (Carapa guianensis) induced structural changes to the mitochondria and kinetoplasts in promastigote forms of L. amazonensis. Oliveira et al.9 also demonstrated activity against promastigotes of L. amazonensis using limonoid-rich oil fractions from andiroba. In our study, we evaluated for the first time the limonoids isolated and identified by spectroscopic techniques against promastigotes of Leishmania amazonensis and L. guyanensis.
CONCLUSIONS Our phytochemical studies showed that the cedar (Cedrela odorata) demolition wood, despite being about forty-five years old, still possessed its secondary metabolites, including the characteristic ones of the species such as tetracylic triterpenes of the cycloartane type, with a mixture of hemiacetal in the side chain in addition to limonoids with an antileishmanial effect. Research related to the search for active compounds from wood derived from demolition has not been disseminated, which indicates a gap to be explored in the search for prototypes as a proposed circular economy model to generate economic and environmental benefits.
SUPPLEMENTARY MATERIAL Supplementary data associated with this article (Figures 1S and Tables 1S-2S) can be found in the online version at http://quimicanova.sbq.org.br as PDF file, with free access.
DATA AVAILABILITY STATEMENT Data supporting the findings of this study are available in the text.
ACKNOWLEDGMENTS The authors would like to thank INCT-Madeiras da Amazônia (MCTI/CNPq/FAPEAM) for financial support, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the scholarship awarded to Henrique Cativo dos Santos and Fundação de Amparo à Pesquisa Estado do Amazonas (FAPEAM) for the productivity grant awarded to Maria da Paz Lima (Call No. 013/2022 - Produtividade - CT&I).
AUTHOR CONTRIBUTIONS Paulo Alan D. Nogueira, Henrique C. dos Santos, Bruno B. Jensen, Antonia Maria R. Franco: investigation, methodology and writing; Claudete C. do Nascimento: investigation, methodology and formal analysis; Pamela Emily T. Iwabuchi: investigation and methodology; Maria da Paz Lima: conceptualization, project administration, writing - original draft and writing - review and editing.
REFERENCES 1. Meliaceae, Cedrela odorata L., https://cncflora.jbrj.gov.br/ficha/9992, accessed in August 2025. 2. Herbário Virtual, https://floradobrasil.jbrj.gov.br/reflora/herbarioVirtual/ConsultaPublicoHVUC/ConsultaPublicoHVUC.do, accessed in August 2025. 3. Instituto Chico Mendes de Conservação da Biodiversidade, 07_-_PORTARIA_MMA_Nº_148_DE_7_DE_JUNHO_DE_2022.pdf, https://www.gov.br/icmbio/pt-br/assuntos/biodiversidade/pan/saiba-mais/documentos-e-downloads/07_-_portaria_mma_no_148_-de_7_de_junho_de_2022.pdf/view, accessed in August 2025. 4. Rondon Neto, R. M.; Lage, C. A.; Bilibio, F.; dos Santos, A. R.; Ambiência 2011, 7, 103. [Crossref] 5. Navarro, C.; Montagnini, F.; Hernández, G.; For. Ecol. Manage. 2004, 192, 217. [Crossref] 6. Da Silva, M. F. G. F.; Agostinho, S. M. M.; de Paula, J. R.; Neto, J. O.; Castro-Gamboa, I.; Filho, E. R.; Fernandes, J. B.; Vieira, P. C.; Pure Appl. Chem. 1999, 71, 1083. [Crossref] 7. Nogueira, T. S. R.; Passos, M. S.; Nascimento, L. P. S.; Arantes, M. B. S.; Monteiro, N. O.; Boeno, S. I. S.; de Carvalho Junior, A.; Azevedo, O. A.; Terra, W. S.; Vieira, M. G. C.; Braz-Filho, R.; Vieira, I. J. C.; Molecules 2020, 25, 5401. [Crossref] 8. Almeida-Souza, F.; Oliveira, I. S. S.; Magalhães, I. F. B.; Taniwaki, N. N.; Calabrese, K. S.; Abreu-Silva, A. L.; Acta Amazonica 2024, 54, e54bc23113. [Crossref] 9. Oliveira, I. S. S.; Tellis, C. J. M.; Chagas, M. S. S.; Behrens, M. D.; Calabrese, K. S.; Abreu-Silva, A. L.; Almeida-Souza, F.; BioMed Res. Int. 2018, 2018, 5032816. [Crossref] 10. Steverding, D.; Sidjui, L. S.; Ferreira, É. R.; Ngameni, B.; Folefoc, G. N.; Mahiou-Leddet, V.; Ollivier, E.; Stephenson, G. R.; Storr, T. E.; Tyler, K. M.; J. Nat. Med.2020, 74, 606. [Crossref] 11. Leishmaniasis: Epidemiological Report for the Americas. No. 12 (December 2023), https://www.paho.org/en/documents/leishmaniasis-epidemiological-report-americas-no12-december-2023, accessed in August 2025. 12. Ministério da Saúde, Secretaria de Vigilância em Saúde, Departamento de Vigilância das Doenças Transmissíveis; Manual de Vigilância da Leishmaniose Tegumentar; Ministério da Saúde: Brasília, 2017. [Link] accessed in August 2025 13. Osorio, E.; Arango, G. J.; Jiménez. N.; Alzate, F.; Ruiz, G.; Gutiérrez, D.; Paco, M. A.; Giménez, A.; Robledo, S.; J. Ethnopharmacol. 2007, 111, 630. [Crossref] 14. Rashed, K.; Hygeia: J. Drugs Med. 2014, 6, 25. [Crossref] 15. Cerda-García-Rojas, C. M.; Hernández-Vidal, H. H.; Joseph-Nathan, P. Magn. Reson. Chem. 1996, 34, 777. [Crossref] 16. Ageta, H.; Arai, Y.; Phytochemistry 1983, 22, 1801. [Crossref] 17. Tanaka, T.; Koyano, T.; Kowithayakorn, T.; Fujimoto, H.; Okuyama, E.; Hayashi, M.; Komiyama, K.; Ishibashi, M.; J. Nat. Prod. 2001, 64, 1243. [Crossref] 18. Ntalli, N. G.; Cottiglia, F.; Bueno, C. A.; Alché, L. E.; Leonti, M.; Vargiu, S.; Bifulco, E.; Menkissoglu-Spiroudi, U.; Caboni, P.; Molecules2010, 15, 5866. [Crossref] 19. Hayasida, W.; Oliveira, L. M.; Ferreira, A. G.; Lima, M. P.; Chem. Nat. Compd. 2017, 53, 312. [Crossref] 20. de Paula, J. R.; Vieira, I. J. C.; Silva, M. F. G. F.; Rodrigues-Filho, E. R.; Fernandes, J. B.; Vieira, P. C.; Pinheiro, A. L.; Vilela, E. F.; Phytochemistry 1997, 44, 1449. [Crossref] 21. Bellone, M. L.; Camero, C. M.; Chini, M. G.; Dal Piaz, F.; Hernandez, V.; Bifulco, G.; De Tommasi, N.; Braca, A.; J. Nat. Prod. 2021, 84, 724. [Crossref] 22. Vasquez-Ruiz, V.; Ramírez-Cisneros, M. Á.; Rios, M. Y.; Magn. Reson. Chem. 2022, 60, 275. [Crossref] 23. Olatunji, L.; Odebunmi, C. A.; Adetunji, A. E.; Future Journal of Pharmaceutical Sciences 2021, 7, 74. [Crossref] 24. Dias, K. K. B.; Cardoso, A. L.; da Costa, A. A.; Passos, M. F.; da Costa, C. E. F.; da Rocha Filho, G. N.; Andrade, E. H. A.; Luque, R.; do Nascimento, L. A. S.; Noronha, R. C. R.; Arabian J.Chem. 2023, 16, 104629. [Crossref] 25. Nogueira, P. A. D.; Santos, H. C.; Iwabuchi, P. E. T.; Jensen, B. B.; Pereira, A. M. R. F.; Nascimento, C. C.; Lima, M. P.; Exatas Online 2022, 13, 24.
Editor handled this article: Jorge M. David |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access