Artigo
| Production and characterization of bio-oil from pyrolysis of biomass waste: a comparative study |
|
Janaina S. MatosI; Flávia S. CunhaII; Soraia T. BrandãoI,III; Maria L. A. da SilvaIII,* I. Programa de Pós-Graduação em Engenharia Química, Escola Politécnica, Universidade Federal da Bahia, 40210-630 Salvador - BA, Brasil Received: 02/20/2025 *e-mail: marialas@ufba.br Faced with resource scarcity and the increasing demand for sustainable solutions, the recovery of agricultural waste presents a promising alternative. This study investigated the pyrolysis of sugarcane straw (PC), brewer waste (BW), and banana tree straw (PB) for the production of bio-oil. The samples were analyzed using thermogravimetric analysis (TG/DTG), X-ray diffraction (XRD), and Fourier-transform infrared spectroscopy (FTIR) to determine their chemical composition and evaluate how the main constituents (cellulose, hemicellulose, and lignin) influenced the properties of the bio-oil. Analytical pyrolysis allowed for the identification of compounds present in the bio-oil. Banana tree straw, which is rich in hemicellulose, produced higher levels of carbon dioxide. In constrast, brewer waste and sugarcane straw, which are rich in lignin, generated greater amounts of phenols and anhydrous sugars. The results demonstrated the potential of pyrolysis for converting agricultural waste into bio-oil, highlighting that the chemical composition of biomass significantly affects the quality and quantity of the products obtained. INTRODUCTION The climate crisis is one of the greatest dilemmas faced by contemporary researchers. Although it occurs naturally, anthropogenic activity is primarily responsible for its intensification. Rapid industrialization and accelerated population growth have increased the consumption of natural resources, establishing a relationship of dependence between oil and technological and economic development.1 The burning of fossil fuels causes the massive emission of greenhouse gases, such as CO, CO2, NOx, and CH4, which, in addition to contributing to global warming, threaten human health due to the reduction in air quality caused by the presence of pollutants dispersed in the atmosphere.2 In this context, due to the environmental problems associated with fossil fuels, the imminent possibility of their depletion, and price fluctuations, it became necessary to diversify the energy matrix, setting precedents for innovation through alternative energy sources. Biomass stands out for its great energy potential, being a cheap and sustainable alternative to fossil-based derivatives, allowing the production of emission-neutral fuels and high-value-added chemical products.3 Biomass encompasses a wide range of materials, including agricultural waste, animal waste, and municipal waste, which are often underutilized, contributing to the accumulation of garbage.4 Lignocellulosic biomasses consist of complex three-dimensional arrangements, composed mainly of hemicellulose, cellulose, and lignin in different proportions, in addition to extractives and ash in the form of inorganic compounds.5 It is estimated that 120 billion tons of lignocellulosic biomass are generated around the globe, with only 3% destined for producing chemicals, bioenergy, and non-food bioproducts.6 When comparing biomass residues with petroleum and its derivatives, it is observed that the former is rich in oxygen, but has lower percentages of hydrogen and carbon. This composition facilitates the obtaining of several chemical products of commercial interest through sustainable routes, using biorefineries. Fast pyrolysis, consists of the thermoconversion of organic material (lignocellulosic biomass) in the absence of oxygen, in an inert atmosphere, at temperatures between 300 and 700 ºC, resulting mainly in bio-oil, biochar, and non-condensable gases.7 The main advantage of this method lies in the significant yield of bio-oil achieved by employing a high heating rate and short residence time of pyrolytic vapors.8 Although the composition of bio-oil represents a challenge for its use as fuel, the diversity of chemical species present makes it attractive. For this reason, bio-oil is considered an important intermediate in the production of chemical products derived from petroleum. Acids, aldehydes, ketones, alcohols, anhydrous sugars, esters, and furans are some of the classes of compounds present in bio-oil in concentrations above 5%, justifying the interest in the separation and large-scale transactions of these products.9 Bio-oils that are rich in aromatics such as benzene, toluene, and xylene, along with phenolic compounds and anhydrous sugars, have been produced from various lignocellulosic sources. These bio-oils offer a promising pathway for the production of biomass-based molecules.10-14 Brazil generates large volumes of waste, which presents significant challenges in terms of management and disposal. As a result, much of the potential of this waste remains untapped. To address this issue, this study proposes the valorization of lignocellulosic waste from sugarcane straw, banana tree straw, and the beer industry, with the goal of producing high-added-value chemicals through fast pyrolysis. Bio-oil and current applications Bio-oil derived from natural lignocellulosic biomass is a dark, viscous liquid with a pungent odor, composed of a complex mixture of about 400 oxygenated organic compounds, including aldehydes, hydrocarbons, ketones, alcohols, esters, carboxylic acids, phenols and sugars.15 During pyrolysis, several reactions such as aromatization, coking, dehydration, dehydrogenation, hydrolysis, isomerization, and retro condensation convert biomass into bio-oil. The pyrolysis process is considered complex due to the various variables involved, which significantly affect the yield of the products. Factors such as residence time, temperature, heating rate, gas flow rate, and biomass composition are determinants for the quality of the bio-oil produced.16 Initial research on bio-oil focused on its application for generating heat and power in diesel and fuel oil engines, highlighting it as a potential substitute for fossil fuels. A major advantage of using bio-oil as fuel is its low emission of pollutant gases, such as CO2, NOx, and SOx. Studies by Castro et al.17 demonstrated that, through the distillation of açai bio-oil, it is possible to obtain gasoline and kerosene with yields between 14 and 42%, presenting itself as an alternative to petroleum refining. Yu et al.18 positively evaluated crude bio-oil obtained from wood chips as a substitute for diesel. Furthermore, Ohra-Aho et al.19 enhanced fast pyrolysis bio-oil for application as marine fuel, expanding its application possibilities. However, despite its environmental appeal, bio-oil has some challenging properties. It has a high moisture content (20-30% for bio-oil, compared to only 0.32% for diesel), low calorific value (13-19 MJ kg-1 for bio-oil versus 40-42 MJ kg-1 for high molecular weight fuels), high acidity (pH 2-5 for bio-oil, pH 9 for diesel), low stability and is susceptible to aging processes.16,20-23 These characteristics limit its direct use as fuel, requiring improvement technologies. Strategies such as co-pyrolysis, catalytic hydrotreatment with modified zeolites, and pretreatments applied to biomass have been considered to deoxygenate bio-oil.24,25 With the advancement of characterization methods, the complex organic composition of bio-oil has proven to be an excellent raw material for the manufacture of green products, such as resins, adhesives, biodegradable films, and binders in the manufacture of bio-asphalt.26-32 Furthermore, bio-oil is not restricted to large industries and energy efficiency; specimens containing acidic, ketonic and alcoholic groups are ideal for agribusiness, giving them fertilizing and pesticide action.
EXPERIMENTAL Preparation of in natura biomass samples The biomasses chosen for this study are abundant due to the strong agro-industrial sector in Bahia, where the research was conducted. Situated in the Northeast region of Brazil, Bahia enjoys ideal climatic conditions for cultivating sugarcane and bananas, resulting in the production of millions of tons of these crops for both domestic and international markets. Additionally, the state is home to two large breweries that significantly contribute to the local economy. During the beer production process, these breweries generate substantial biomass residues in the form of bagasse. Utilizing these residues as raw materials to produce high-value products represents an innovative and sustainable approach, underscoring the potential of local biomass to foster a more diversified and environmentally responsible economy. Sugarcane straw (sample PC), banana straw (sample PB) and brewer waste (sample BW) biomass collected in the Northeast region of Brazil were washed in running water to remove surface impurities. They were then dried at 100 ºC for 24 h, crushed and sieved (32-60 mesh). Chemical characterization The composition of the biomass was determined following methodologies recommended in the literature for the characterization of lignocellulosic materials. The extractive content was determined according to the adapted American Society for Testing and Materials (ASTM) D1105-2133 method, using ethanol and water as solvents. The in natura biomass samples were hydrolyzed at 45 ºC, in an acidic medium containing H2SO4 (4%), and then had their cellulose and lignin contents quantified using the procedure described by Gouveia et al.34 To determine the insoluble lignin content (Klason), the methodology described by Technical Association of the Pulp and Paper Industry (method TAPPI T 222 om-88)35 was followed. Additionally, the soluble lignin content was determined according to Ruwoldt et al.,35 in agreement with TAPPI T UM 250,35 whose absorbance measurement was carried out at 280 nm using distilled water as a blank. The ash content was determined by calculating the difference in mass between the crucibles that had previously calcined (for 3 h at 800 ºC) following the ASTM E1755-01.36 X-ray diffraction (XRD) X-ray diffraction analyses were performed using a Shimadzu X-ray diffractometer, model XRD-6100, with copper radiation (Kα) (λ = 1.5418 Å), in the range of 10 to 80º, with a speed of 10 degrees min-1. Thermogravimetric analysis (TG) Thermogravimetric analyses were conducted on Shimadzu DTG-60 equipment, with a heating ramp of 20 ºC min-1 up to 700 ºC, in an inert atmosphere (N2), with a flow rate of 20 mL min-1. Fourier transform infrared spectroscopy (FTIR) Fourier transform infrared spectroscopy analyses were performed using a PerkinElmer 100 FTIR spectrophotometer, model Spectrum 100S, operating in the region of 4000 to 450 cm-1, with a resolution of 4 cm-1 and 32 accumulations per spectrum. Analytical pyrolysis Analytical pyrolysis of biomass samples was carried out in a Frontier micropyrolyzer model EGA/PY-3030D coupled to a gas cromatography-mass spectrometry (GC-MS) Shimadzu model QP-2020NX. For the analysis, 0.5 mg of each biomass was used and subjected to a temperature of 550 ºC in the micropyrolyzer. The volatiles produced through pyrolysis were directed to a gas chromatograph whose injector temperature was maintained at 250 ºC. Helium was used as carrier gas with a flow of 1 mL min-1 through an Rtx-1701 GC 195 capillary column (60 m × 0.25 mm × 0.25 µm). The chromatographic oven was initially maintained for 4 min at 45 ºC and subsequently heated to 235 ºC at a rate of 3 ºC min-1, remaining at this temperature for 7 min. The chromatogram peaks were obtained with 80% similarity using the National Institute of Standards and Technology (NIST 14) database.
RESULTS AND DISCUSSION Chemical characterization Table 1 shows the contents of hemicellulose, cellulose, lignin, ash, and extractives present in the three biomasses studied, which are in agreement with values already reported in the literature,37 except for the content of extractives present in the PB biomass. The relative cellulose content was higher in the PC biomass, followed by PB and BW. Cellulose is composed of D-glucopyranose units, linked by β-1,4 glycosidic bonds, whose pyrolysis releases anhydrous sugars, furans, furanones, benzenes (phenols and catechols), cyclopentanones and light molecules (acetaldehyde, methylglyoxal, and hydroxy acetaldehyde) through the cleavage of glycosidic bonds and dehydration reactions.38 On the other hand, BW biomass presented a higher content of lignin, a complex phenolic polymer composed of three main units: guaiacyl propanol (G), syringyl propanol (S), and p-hydroxyphenyl propanol (H).39 During pyrolysis, lignin contributes positively to the release of phenols, suggesting that biomasses rich in this constituent are the most suitable for this purpose.40
According to the results obtained, PB had the highest hemicellulose content, followed by PC and BW, respectively. Hemicellulose is a polysaccharide composed of carbohydrate groups that vary according to the type of biomass considered.41 Its chain contains several sugars, including D-xylopyranose, D-galactopyranose, L-arabinofuranose, D-mannopyranose, D-glucopyranosyluronic acid, and D-galactopyranosyluronic acid, joined by β-(1,4) bonds.42 Some studies on hemicellulose pyrolysis provide valuable information on the composition of bio-oil derived from this fraction. Reyes et al.43 observed that, unlike lignin bio-oil, which is rich in phenolic compounds, bio-oil from hemicellulose has a high concentration of acids, such as acetic and propanoic acids, as well as alcohols and ketones. Previous studies44 indicate that biomasses rich in cellulose and hemicellulose produce high bio-oil yields since the thermal decomposition of these fractions occurs at lower temperatures than lignin. On the other hand, the relative ash content was higher in PC biomass. Ash in biomass is composed of alkali and alkaline earth metals (AAEM), which catalyze the cleavage of levoglucosan through the opening of the cellulose ring, producing low molecular weight molecules such as aldehydes, ketones, and acids, thus decreasing the yield of this sugar at high temperatures.45 The high reactivity exhibited by AAEM can be justified by their Lewis acid character, which intensifies the dehydration reactions of sugars during pyrolysis.46 Comparing the relative acidity of these species, alkaline earth metals (Ca2+, Mg2+) are more acidic than the ions of their Group 1 neighbors (Na+, K+), selectively leading to the depolymerization of cellulose and promoting the conversion of anhydrous sugars into furfural.47 Extractives are present in lignocellulosic biomass in small amounts, ranging from 5 to 30% in dry mass of biomass. These extractives include saponins, phenolics, starch, waxes, pigments, simple sugars, and proteins.48 Among the samples analyzed, the highest relative content of extractives was observed in the PB sample, a value considered high compared to the other samples and to the values found in the literature for pseudo stem.49 This result can be attributed to the seasonality of the residue, the cultivation conditions, and the different regions of origin.50 Extractives encompass several distinct compounds in terms of polarity and solubility; hydrophilic ones are soluble in water and/or polar solvents, consisting of a mixture of sugars, acids, proteins, pigments and terpenes, while lipophilic ones include molecules soluble in nonpolar solvents, such as fatty acids, resins and sterols.51 Few studies investigated the influence of extractives on the composition of bio-oil, however, researchers from the group of Sampaio et al.52 related the significant production of CO2 to extractives to the detriment of the formation of monosaccharides, a group of which D-allose is part. Guo et al.53 observed that extractives favor the production of acids, such as acetic acid. Additionally, Clemente-Castro et al.54 noted that extractives also contribute to increasing the yield of aromatic compounds. Extractives have a positive impact on the reactivity of other biomass constituents, as well as on the yield of pyrolysis products.55 According to Gogoi et al.,56 this biomass fraction contributes to increasing the yield of bio-oil, while reducing the production of biochar. Due to the organic nature of this fraction, it is common for bio-oil from biomasses rich in extractives to present a hydrophilic and a hydrophobic phase, the latter being poor in oxygenates, more viscous and with a higher calorific value (HHV) compared to the former. X-ray diffraction (XRD) As shown in Figure 1, intense diffraction peaks at 2θ = 22º were observed in all three samples, corresponding to the crystallographic planes of cellulose I (002), which is the native form of cellulose.42 Pyrolysis of lignocellulosic biomasses containing cellulose I can result in the production of bio-oil rich in anhydrous sugars and their derivatives, furfural, furans, and light oxygenated compounds, such as hydroxyacetaldehyde and 1-hydroxy-2-propanone.57 In samples PC and PB, peaks at 2θ = 15º were also identified, corresponding to the crystallographic planes of cellulose I (110).58
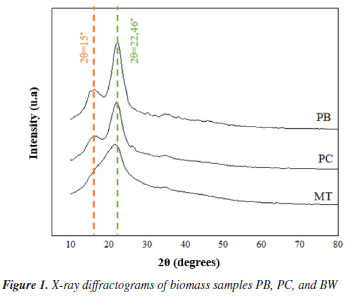
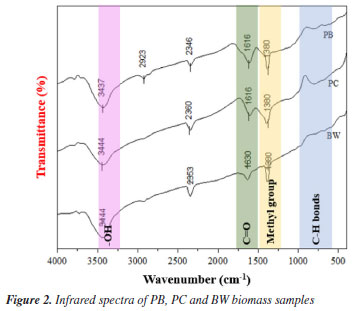
The PC biomass presented a higher cellulose content than the other biomasses studied (Table 1), showing a more intense peak of crystallin cellulose at 22.46º. Cellulose has amorphous and crystalline regions, and its crystallinity impacts physical properties such as elasticity, thermal stability, absorptive capacity, and rigidity.59 Biomasses with amorphous characteristics tend to be more reactive than crystalline ones, becoming more susceptible to complex thermal degradation reactions, such as pyrolysis, and facilitating accessibility to chemical and biological treatments.60 Fourier transform infrared spectroscopy (FTIR) Figure 2 shows the FTIR spectra of the PB, PC, and BW samples, evidencing the presence of bands characteristic of lignocellulosic biomass. Absorption bands characteristic of C-H bonds in carbon belonging to aromatic rings (900-690 cm-1) present in lignin were identified, as well as bands characteristic of the methyl group (1450-1373 cm-1), typical of lignin, cellulose, and hemicellulose. In addition, bands at 1635 and 1728 cm-1 were observed, possibly attributed to carbonyls (C=O) present in cellulose and lignin, and an O-H band at 3441 cm-1, which may be due to phenolic or alcoholic groups associated with lignin.61
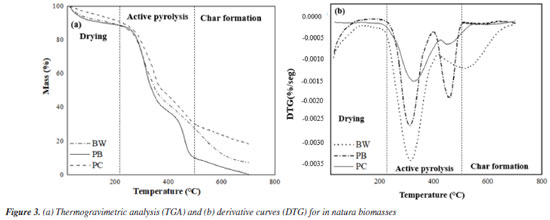
Thermogravimetric analysis (TG/DTG) The TGA curves (Figure 3a) obtained for the biomasses exhibit four main stages: (i) water evaporation and removal of extractives, from room temperature to approximately 210 ºC; (ii) degradation of cellulose and hemicellulose between 210 and 402 ºC; (iii) decomposition of cellulose and part of the lignin between 402 and 506 ºC; (iv) decomposition of the remaining lignin from 506 to 629 ºC. The delimited temperature ranges are similar to those found for the decomposition of sisal residues and for three different lignocellulosic biomasses: cedar sawdust, coffee processing residues, and rice straw.62
The DTG curves (Figure 3b) can be divided into three distinct events: drying, active pyrolysis zone, and char formation (or carbonization), all common to lignocellulosic biomasses.63 In the drying stage, water and extractives are released, while the active pyrolysis stage is characterized by the decomposition of hemicellulose (peak or left "shoulder"), as well as by the maximum decomposition of all samples with the formation of large amounts of volatiles.64,65 In the curve referring to PC biomass, this stage occurs between 328 and 455 ºC, for BW, between 314 and 524 ºC, and for PB, between 300 and 500 ºC. In carbonization, where temperatures are higher than in pyrolysis, the remaining biomass decomposes slowly, producing charcoal. This process is reflected in the DTG curves as a slight loss of mass, with a tendency to become increasingly linear.66 From the TG/DTG curves, it is also possible to determine an optimal temperature range for pyrolysis. Considering that fast pyrolysis, ideal for bio-oil production, is performed at intermediate temperatures, it can be inferred that the temperature range of 450 to 550 ºC is the most suitable for pyrolysis of the biomasses under study. This range ensures a good loss of mass (approximately 70% for the BW and PC samples and 90% for the PB sample) and maximizes the yield of the desired liquid phase. Analytical pyrolysis Figure 4 shows the distribution of compounds present in bio-oils originating from the analytical pyrolysis of the different biomasses studied in this work. The components of bio-oils are distributed among acids, alcohols, aldehydes, anhydrous sugars, esters, furans, hydrocarbons, ketones, phenolic compounds and others, including N-components and P-components. Tables 1S to 3S (Supplementary Material) provide the complete list of chemical components identified in the provided bio-oil samples.
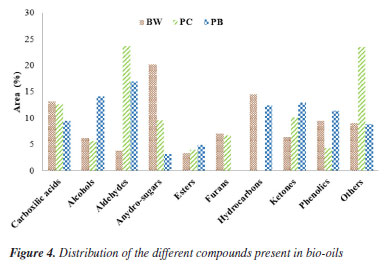
The PC and PB biomasses favored the production of aldehydes, with relative areas of 24 and 17%, respectively. On the other hand, the low production of aldehydes in the bio-oil of the BW sample stands out, at around 4%. The highest production of alcohols was observed for the PB sample (14%), followed by BW (6%) and PC (5%). Aliphatic alcoholic compounds, such as 1-propanol, 1,3-propanediol, and methanol, as well as cyclic compounds, such as 6-oxa-bicyclo[3.1.0]hexan-3-ol and cyclopentanol, were identified. The PB sample also produced the highest ketone content (14%), compared to PC (10%) and BW (6%). Alcohols, aldehydes, and ketones are formed via decarbonylation, dehydration, ring opening, and C-C bond cleavage reactions, all catalyzed by cellulose and hemicellulose.67 The relationship between composition and yield of oxygenated compounds is associated with the relative hemicellulose content, being evident in the PB sample, which occupies the second position in relative amount of cellulose, but the first in relative hemicellulose, as shown in Figure 4, in which the PB sample stands out in the production of oxygenated compounds. The presence of oxygenated species such as carboxylic acids, ketones, and aldehydes in bio-oil is often viewed negatively, due to a series of complications. For example, organic acids such as formic and acetic acids harm the pH of bio-oil, making its storage and transport difficult, while phenols and aldehydes impact stability levels and, consequently, the fluidity of the liquid.68 However, some compounds from these groups are of commercial interest, finding applications in the food and pharmaceutical industries. One example is 2,3-butanedione, a ketone detected in bio-oil samples with a relative area of approximately 2% (Tables 1S-3S). This substance is considered non-toxic by the United States Food and Drug Administration (US FDA) and is used as a food additive in dairy products to provide a buttery flavor.69 In the pharmaceutical industry, 2-cyclopenten-1-one, 2-hydroxy-3-methyl stands out, which, although identified with a small area relative to the PB bio-oil sample (1.57%), has a high added value due to its excellent anti-inflammatory activity.70 Acrolein (2-propenal, relative area of 1.34%), belonging to the aldehyde class, stands out as an intermediate in the manufacture of chemical products, consolidating the global market with a value of 1.52 billion dollars, with projections to reach 1.75 billion dollars by 2026.71 Methylglyoxal (Table 2S, relative area 4.08%) acts as a precursor in the generation of various flavorings. When reacting with amino acids such as valine, leucine, isoleucine, methionine and phenylalanine, it forms compounds such as isobutanal, isovaleraldehyde, 2-methyl butanal, methional and phenylacetaldehyde, respectively, originating new flavors, including roasted meat, roasted nuts, malt, cabbage, cereals, aroma cherry and rose.58 Despite the added value of these compounds, most studies on oxygenated compounds present in pyrolysis bio-oil focus on their conversion, rather than their recovery. The most promising path for converting aldehydes and congeners into hydrocarbons is hydrodeoxygenation (HDO).59 This process occurs at high temperatures, generally between 300-600 ºC under a hydrogen atmosphere (> 30 bar), through dehydration, decarboxylation, decarbonylation, alkylation, oligomerization, and hydrogen transfer reactions.60 Several catalysts have been investigated for this purpose, including noble metal oxides, zeolites, bifunctional catalysts, and clays.64 Among these, acidic zeolites, in particular HZSM-5, are the most used due to their shape selectivity for target molecules, providing high yield of hydrocarbons.72 Selectivity results from the mass transfer effect, which excludes reactant molecules larger than the pore size distribution, which can vary between 0.5-1.2 nm.73 The production of anhydrous sugars, such as 1,6-anhydro-beta-D-glucopyranose and levoglucosan, stands out in BW (ca. 20%) and PC (ca. 10%) biomasses. These sugars are products of the thermal degradation of cellulose, resulting from the fragmentation of H-C-O-H bonds, abundant in the structure of native cellulose.74 The breaking of the glycosidic bonds (1 → 4) followed by dehydration results in a high yield of levoglucosan (LG).75 Considered a versatile molecule, LG is widely used in the production of pharmaceuticals, surfactants, agrochemicals, polymers, and fuels, in addition to being an excellent precursor for the production of alcohols and acids through fermentation.76 The conventional LG synthesis method uses D-glucose as the starting compound. Due to its regioselectivity, the hydroxyl linked to carbon 6 is preferentially attacked, originating a new aromatic compound. However, this route is expensive and complex, due to the numerous protections and deprotections steps of functional groups and the activation of anomeric sugar centers.77 The complexity of the synthesis is reflected in the high market price of the product, which varies between USD 10,000-50,000 per metric ton.78 In recent years, levoglucosan has received special attention because it is the main degradation product of cellulose, the most abundant polymer in biomass. Its degradation mechanism is well elucidated, and it is identified in bio-oil in concentrations greater than 50%, offering an alternative path for the production of monomeric sugars, such as glucose, a precursor for biomass-derived fuels.79 However, the production of sugars via pyrolysis faces challenges due to the inhibitory nature of hemicellulose, which explains the low yield observed in PB biomass (3%), which had the highest hemicellulose content among the samples studied (Table 1). The study of this carbohydrate generally takes as a reference the structure of xylan, composed mainly of pentoses that cannot be converted into anhydrides via intermolecular dehydration due to the absence of the hydroxyl group.66 BW and PB biomasses resulted in the production of bio-oil rich in hydrocarbons, with 14 and 12% in area, respectively. Biomass constituents are converted into hydrocarbons through dehydration, cyclization, alkylation, isomerization, dehydration, decarbonylation, decarboxylation, oligomerization, and disproportionation reactions.60 Alkali metals (AAEMs) present in ash favor cracking and dehydration reactions, producing volatiles and reducing the yield of hydrocarbons in bio-oil.80 As shown in Table 1, PC biomass had the highest ash content and, consequently, the highest AAEMs content, which justifies the absence of hydrocarbons in the bio-oil derived from this biomass. Zhang et al.81 observed a similar trend when investigating the impact of alkali metals on the pyrolysis of rice husks, concluding that AAEMs do not promote the formation of hydrocarbons, but rather of oxygenated compounds (aldehydes, ethers, and ketones). The main interest in hydrocarbon-rich bio-oils is related to their potential as fuel. Fuels derived from petroleum refining contain hydrocarbons with different carbon numbers, giving them varied uses. For example, lighter fractions are used to generate heat and energy for medium-sized transport; diesel provides energy for large vehicles; and kerosene is widely used in the aerospace sector.82 The highest production of carboxylic acids was observed for BW biomass (14%), followed by PC (13%) and PB (9%). These acids are released during the decomposition of hemicellulose, generally at temperatures between 300 and 400 ºC through cleavage reactions of the acetyl side chain, cracking, retro-aldol condensation and dehydration.83 However, recent studies81 indicate that acids with lower molar mass, such as acetic, can be formed by the decomposition of lignin at elevated temperatures. According to the data presented in Table 1, BW biomass has the highest relative lignin content and lowest hemicellulose content, while PB has the highest relative hemicellulose content. Given the tendency of hemicellulose to release acids, it was expected that PB would provide a higher yield of carboxylic acids, which was not observed. The key point in the discussion is the ash content, rich in alkaline and alkaline earth metals, present in the composition of CP at around 1.34% (Table 1). According to Alcazar-Ruiz et al.,84 these metals quickly convert carboxylic acids into esters, thus reducing their content in bio-oil samples. Acetic and hexadecanoic acids were also identified (Table 1S), with individual relative areas varying between 2.41 and 3.54%. Among the carboxylic acids obtained by pyrolysis, acetic acid is the most important. In addition to acting as a reagent and solvent, it has commercial application in the production of polyethylene terephthalate, cellulose acetate, and vinyl acetate, used in various areas, such as automobiles, food, packaging polymers and paints.85 Few studies have focused on the production and recovery of acetic acid by pyrolysis. The oldest of them focused on recovering the acid contained in fast pyrolysis bio-oil, obtaining 90% acetic acid by weight.86 The production of phenolic compounds by PB and BW biomass was 14 and 11%, respectively, while for PC it was less than 5%. This result can be explained based on the composition of the raw materials. Among the biomasses considered, PC has the highest relative content of ash, which contains alkaline and alkaline earth metals, which reduce the formation of phenolic compounds.87 The differences observed between PB and BW in the production of phenolic compounds are largely attributed to the lignin content, which is 17 and 27%, respectively, for these biomasses. Functional groups such as -OCH3 and -OH linked to aromatic rings or side chains of its basic units are identified in the structure of lignin.88 Lignin pyrolysis is divided into three stages: initial, primary, and carbonization. The primary stage, which occurs between 400 and 600 ºC, is the most significant, culminating in the generation of aromatic hydrocarbons and phenolic compounds, formed by the interaction of H radicals with those arising from the scission of the Cβ-O and Cα-O bonds of lignin.89 This reaction can begin via homolytic cleavage of the lignin glycosidic bond (β-O-4 or α-O-4) or by intra- or intermolecular transfer. However, homolytic reactions, such as concerted elimination and ene-type reactions, produce dimers that, with increasing temperature, fragment into small monomers, with alkoxy-phenols being the most common representatives of this group.88 In the bio-oils analyzed, several phenolic compounds were identified, such as cresols, benzene, 2,6-dimethoxy-4-(2-propenyl), 2-methoxy-1,3,4-trimethyl- and phenol,4-ethyl (Tables 1S-3S). The interest in the production and recovery of phenolic compounds lies in their antioxidant and antimicrobial properties, in addition to their application as additives and raw materials in the production of resins, dyes, plastics, and pesticides.90 In human health, these compounds exhibit numerous benefits, since, by preventing the oxidative process, they inhibit the formation of free radicals responsible for serious diseases, such as cancer, vascular diseases, and even neurological disorders.91 Analogous to hydrocarbons, high yields of phenolics are achieved selectively through catalytic pyrolysis. Ma et al.92 examined selective deoxygenation with silica-supported catalysts (Ni/SiO2, Ca/SiO2, Ni-Ca/SiO2), observing an increase in the production of phenolic compounds when Ni/SiO2 and Ni-Ca/SiO2 were introduced into the medium reactional. Using a metal catalyst supported on zeolite (Ni/HZSM-5), Ouedraogo et al.93 found a 47.8% increase in selectivity for phenolic compounds (CF) compared to the uncatalyzed reaction. It was observed that PB-derived bio-oil did not produce furans, while approximately 7% of furans were made in both BW and PC bio-oil. The possible reason for the absence of furans in the composition of PB bio-oil lies in the interaction between cellulose and hemicellulose, which prevents dehydration reactions and ring opening of C6 monomers from occurring, hindering the formation of these molecules.94,95 A similar phenomenon was observed by Zhang et al.96 when investigating the effect of multi-component interactions on the formation of bio-oil, where hemicellulose contributed to the generation of pyrans to the detriment of the formation of phenolics and furans, by promoting the depolymerization and dehydration of cellulose.
CONCLUSIONS Fast pyrolysis of sugarcane straw, brewery residue, and banana tree straw was investigated in this study. Previous characterization of the biomasses by XRD and FTIR indicated the presence of cellulose, hemicellulose, and lignin, with sugarcane straw showing lower crystallinity and greater potential for thermal degradation. Thermogravimetric (TG) analysis revealed that the temperature range between 400 and 550 ºC was the most suitable for bio-oil production. The composition of the bio-oil varied according to the biomass used. Banana tree straw, rich in hemicellulose, generated mainly CO2, while brewery residue and sugarcane straw, with higher lignin content, produced significant amounts of phenols and anhydrous sugars. The presence of alkaline and alkaline earth metals in banana tree straw inhibited the formation of hydrocarbons. The results demonstrate the potential of pyrolysis for the production of bio-oil from agricultural waste, with the composition of the biomass directly influencing the composition of the bio-oil. However, to optimize the production of high-value-added compounds, such as phenols and hydrocarbons, additional investigations are necessary, such as the use of catalysts and the optimization of pyrolysis conditions.
SUPPLEMENTARY MATERIAL Complementary material for this work is available at http://quimicanova.sbq.org.br/, as a PDF file, with free access.
DATA AVAILABILITY STATEMENT All data are available in the text.
ACKNOWLEDGMENTS The authors thank Programa Institucional de Bolsas de Iniciação em Desenvolvimento Tecnológico e Inovação (PIBIC) from Universidade Federal da Bahia (UFBA), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPQ), and Programa de Graduação em Engenharia Química (PPEQ-UFBA/UNIFACS) for their support. They also acknowledge Fundação de Amparo à Pesquisa do Estado da Bahia (FAPESB) for Scientific Initiation and Master's scholarships, as well as PROPCI-PROPG/UFBA (notice No. 007/2022-JOVEMPESQ) and the Ânima Institute for research support.
AUTHOR CONTRIBUTIONS Janaina S. Matos was responsible for investigation, data curation, formal analysis, validation, software, writing (original draft, review and editing); Flávia S. Cunha for methodology, conceptualization, data curation, software, writing (review and editing); Maria L. A. da Silva for project administration, resources, visualization, writing (review and editing); Leila M. A. Campos for project administration, resources, visualization, writing (review and editing); Soraia T. Brandão for conceptualization, funding acquisition, resources.
REFERENCES 1. Rezazadeh, A. A.; Avami, A.; Baghshah, M. S.; Energy Build 2024, 310, 114103. [Crossref] 2. Ning, P.; Yang, G.; Hu, L.; Sun, J.; Shi, L.; Zhou, Y.; Wang, Z.; Yang, J.; Biotechnol. Biofuels 2021, 14, 102. [Crossref] 3. Banu, J. R.; Preethi; Kavitha, S.; Tyagi, V. K.; Gunasekaran, M.; Karthikeyan, O. P.; Kumar, G.; Fuel 2021, 302, 121086. [Crossref] 4. Makepa, D. C.; Chihobo, C. H.; Biomass Convers. Biorefin. 2025, 15, 11397. [Crossref] 5. Mujtaba, M.; Fraceto, L. F.; Fazeli, M.; Mukherjee, S.; Savassa, S. M.; de Medeiros, G. A.; Pereira, A. E. S.; Mancini, S. D.; Lipponen, J.; Vilaplana, F.; J. Cleaner Prod. 2023, 402, 136815. [Crossref] 6. Tamilselvan, R.; Selwynraj, A. I.; Anaerobe 2024, 85, 102815. [Crossref] 7. Vikram, S.; Rosha, P.; Kumar, S.; Energy Fuels 2021, 35, 7406. [Crossref] 8. Pagano, M.; Hernando, H.; Cueto, J.; Cruz, P. L.; Dufour, J.; Moreno, I.; Serrano, D. P.; Chem. Eng. J. 2023, 454, 140206. [Crossref] 9. Sarchami, T.; Batta, N.; Berruti, F.; Biofuels, Bioprod. Biorefin. 2021, 15, 1912. [Crossref] 10. Téllez, J. F.; Silva, M. P.; Simister, R.; Gomez, L. D.; Fuertes, V. C.; De Paoli, J. M.; Moyano, E. L.; J. Anal. Appl. Pyrolysis 2021, 156, 105105. [Crossref] 11. Jiang, M.; Su, Y.; Qi, P.; Zhang, S.; Xiong, Y.; Biomass Convers. Biorefin. 2024, 14, 28947. [Crossref] 12. Tian, H.; Zhu, R.; Chen, L.; Wang, J.; Cheng, Y.; Ind. Crops Prod. 2023, 204, 117314. [Crossref] 13. Liu, P.; Jiang, Z.; Zeng, Y.; Wang, Y.; Zeng, C.; Tu, X.; Yan, K.; Ind. Crops Prod. 2024, 216, 118768. [Crossref] 14. Fardhyanti, D. S.; Megawati; Chafidz, A.; Prasetiawan, H.; Raharjo, P. T.; Habibah, U.; Abasaeed, A. E.; Biomass Convers. Biorefin. 2024, 14, 217. [Crossref] 15. Lachos-Perez, D.; Martins-Vieira, J. C.; Missau, J.; Anshu, K.; Siakpebru, O. K.; Thengane, S. K.; Morais, A. R. C.; Tanabe, E. H.; Bertuol, D. A.; Analytica 2023, 4, 182. [Crossref] 16. Vuppaladadiyam, A. K.; Vuppaladadiyam, S. S. V.; Sahoo, A.; Murugavelh, S.; Anthony, E.; Bhaskar, T.; Zheng, Y.; Zhao, M.; Duan, H.; Zhao, Y.; Antunes, E.; Sarmah, A. K.; Leu, S. Y.; Sci. Total Environ. 2023, 857, 159155. [Crossref] 17. de Castro, A. D. R.; Ribeiro, H. J. S.; Guerreiro, L. H. H.; Bernar, L. P.; Bremer, J. S.; Santo, M. C.; Almeida, H. S.; Duvoisin Junior, S.; Borges, L. E. P.; Machado, N. T.; Ribeiro, S.; Energies 2021, 14, 3713. [Crossref] 18. Yu, Y.; Mei, D.; Yan, Z.; Wang, C.; Feng, P.; Wei, N.; J. Energy Inst. 2025, 120, 102078. [Crossref] 19. Ohra-Aho, T.; Oasmaa, A.; Koponen, P.; Nyyssönen, S.; Tuominen, V.; Aakko-Saksa, P.; Energy Fuels 2024, 38, 19566. [Crossref] 20. Rajamohan, S.; Suresh, S.; Mallinathan, S.; Harigopal, A.; Nguyen, V. N.; Engel, D.; Forruque Ahmed, S.; Hieu Le, T.; Sustainable Energy Technologies and Assessments 2022, 52, 102202. [Crossref] 21. Lindfors, C.; Kuoppala, E.; Oasmaa, A.; Solantausta, Y.; Arpiainen, V.; Energy Fuels 2014, 28, 5785. [Crossref] 22. Micayabas, K. C. C.; Tingalan, D. R.; Pacturan, K. N.; Singgil, D. J. B.; Saldo, I. J. P.; Casas, R. Y.; Peñafiel-Dandoy, M. J.; Am. J. Energy Res. 2023, 11, 117. [Crossref] 23. Musurmanov, R. K.; Utaev, S. A.; Kasimov, I. S.; IOP Conference Series: Earth and Environmental Science 2021, 868, 012044. [Crossref] 24. Chaihad, N.; Karnjanakom, S.; Abudula, A.; Guan, G.; Resour. Chem. Mater. 2022, 1, 167. [Crossref] 25. Terry, L. M.; Li, C.; Chew, J. J.; Aqsha, A.; How, B. S.; Loy, A. C. M.; Chin, B. L. F.; Khaerudini, D. S.; Hameed, N.; Guan, G.; Sunarso, J.; Carbon Resour. Convers. 2021, 4, 239. [Crossref] 26. Özbay, G.; Ayrilmis, N.; Ind. Crops Prod. 2015, 66, 68. [Crossref] 27. Luo, Z.; Zhu, X.; Deng, J.; Gong, K.; Zhu, X.; J. Hazard Mater. 2021, 420, 126570. [Crossref] 28. Asafu-Adjaye, O. A.; Street, J.; Bensode, A.; Auad, M. L.; Peresin, M. S.; Adhikari, S.; Liles, T.; Via, B. K.; Polymers 2022, 14, 1244. [Crossref] 29. Pradeep, S. V.; Kandasubramanian, B.; Sidharth, S.; J. Adhes. 2023, 99, 2145. [Crossref] 30. Yu, Y.; Li, C.; Jiang, C.; Chang, J.; Shen, D.; Polymers 2022, 14, 1352. [Crossref] 31. Hidalgo, P.; Salgado, L.; Ibacache, N.; Hunter, R.; Polymers 2023, 15, 1895. [Crossref] 32. Celikbag, Y.; Nuruddin, M.; Biswas, M.; Asafu-Adjaye, O.; Via, B. K.; J. Adhes. Sci. Technol. 2020, 34, 2743. [Crossref] 33. ASTM D1105-21: Standard Test Method for Preparation of Extractive-Free Wood, Pennsylvania, 2021. 34. Gouveia, E. R.; do Nascimento, R. T.; Souto-Maior, A. M.; Rocha, G. J. M.; Quim. Nova 2009, 32, 1500. [Crossref] 35. Technical Association of the Pulp and Paper Industry (TAPPI) T222: Acid-Insoluble Lignin in Wood and Pulp, Atlanta, 2002. [Link] accessed in September 2025; Technical Association of the Pulp and Paper Industry (TAPPI) U. M. 250: Acid-Soluble Lignin in Wood and Pulp, Atlanta, 1991; Ruwoldt, J.; Tanase-Opedal, M.; Syverud, K.; ACS Omega 2022, 7, 46371. [Crossref] 36. ASTM E1755-01: Standard Test Method for Ash in Biomass, Pennsylvania, 2020. 37. Richard, E. N.; Hilonga, A.; Machunda, R. L.; Njau, K. N.; 3 Biotech 2020, 10, 542. [Crossref] 38. Ansari, K. B.; Arora, J. S.; Chew, J. W.; Dauenhauer, P. J.; Mushrif, S. H.; Ind. Eng. Chem. Res. 2019, 58, 15838. [Crossref] 39. Sethupathy, S.; Morales, G. M.; Gao, L.; Wang, H.; Yang, B.; Jiang, J.; Sun, J.; Zhu, D.; Bioresour. Technol. 2022, 347, 126696. [Crossref] 40. Hu, R.; Zhao, Y.; Tang, C.; Shi, Y.; Luo, G.; Fan, J.; Clark, J. H.; Zhang, S.; Engineering 2023, 27, 178. [Crossref] 41. Faleeva, Y. M.; Lavrenov, V. A.; Zaichenko, V. M.; Biomass Convers. Biorefin. 2024, 14, 14519. [Crossref] 42. Rao, J.; Lv, Z.; Chen, G.; Peng, F.; Prog. Polym. Sci. 2023, 140, 101675. [Crossref] 43. Reyes, L.; Abdelouahed, L.; Mohabeer, C.; Buvat, J. C.; Taouk, B.; Energy Convers. Manage. 2021, 244, 114459. [Crossref] 44. Sakulkit, P.; Palamanit, A.; Dejchanchaiwong, R.; Reubroycharoen, P.; J. Environ. Chem. Eng. 2020, 8, 104561. [Crossref] 45. Silveira Junior, E. G.; Silveira, T. C.; Perez, V. H.; Justo, O. R.; David, G. F.; Fernandes, S. A.; J. Energy Inst. 2022, 101, 254. [Crossref] 46. Chen, Z.; Wang, Y.; Cheng, H.; Zhou, H.; Ind. Crops Prod. 2022, 187, 115335. [Crossref] 47. Li, J.; Bai, X.; Fang, Y.; Chen, Y.; Wang, X.; Chen, H.; Yang, H.; Combust. Flame 2020, 215, 1. [Crossref] 48. Giudicianni, P.; Gargiulo, V.; Grottola, C. M.; Alfè, M.; Ferreiro, A. I.; Mendes, M. A. A.; Fagnano, M.; Ragucci, R.; Energy Fuels 2021, 35, 5407. [Crossref] 49. Abdullah, N.; Mohd Taib, R.; Mohamad Aziz, N. S.; Omar, M. R.; Md Disa, N.; Heliyon 2023, 9, e12940. [Crossref] 50. Martins, J. P. G.; Setter, C.; Ataíde, C. H.; de Oliveira, T. J. P.; Magriotis, Z. M.; Biomass Bioenergy 2021, 149, 106095. [Crossref] 51. Smit, A. T.; van Zomeren, A.; Dussan, K.; Riddell, L. A.; Huijgen, W. J. J.; Dijkstra, J. W.; Bruijnincx, P. C. A.; ACS Sustainable Chem. Eng. 2022, 10, 6012. [Crossref] 52. Sampaio, T. Q. S.; Cunha, F. S.; Campos, L. M. A.; Pires, C. A. M.; J. Anal. Appl. Pyrolysis 2024, 178, 106417. [Crossref] 53. Guo, Z.; Zhang, K.; Zhang, Q.; Fu, L.; Liu, Z.; Kong, Z.; Wang, L.; Liu, C.; Hua, L.; Li, B.; J. Anal. Appl. Pyrolysis 2022, 167, 105650. [Crossref] 54. Clemente-Castro, S.; Palma, A.; Ruiz-Montoya, M.; Giráldez, I.; Díaz, M. J.; J. Anal. Appl. Pyrolysis 2022, 162, 105457. [Crossref] 55. Maaoui, A.; Trabelsi, A. B. H.; Ben Abdallah, A.; Chagtmi, R.; Lopez, G.; Cortazar, M.; Olazar, M.; J. Energy Inst. 2023, 108, 101242. [Crossref] 56. Gogoi, D.; Kumar, M.; Lakshmi, Y. G.; Bioenergy Res. 2023, 16, 1417. [Crossref] 57. Guedes, R. E.; Luna, A. S.; Torres, A. R.; J. Anal. Appl. Pyrolysis 2018, 129, 134. [Crossref] 58. Zheng, J.; Guo, H.; Ou, J.; Liu, P.; Huang, C.; Wang, M.; Simal-Gandara, J.; Battino, M.; Jafari, S. M.; Zou, L.; Ou, S.; Xiao, J.; Trends Food Sci. Technol. 2021, 107, 201. [Crossref] 59. Lahijani, P.; Mohammadi, M.; Mohamed, A. R.; Ismail, F.; Lee, K. T.; Amini, G.; Energy Convers. Manage. 2022, 268, 115956. [Crossref] 60. Shahbeik, H.; Shafizadeh, A.; Gupta, V. K.; Lam, S. S.; Rastegari, H.; Peng, W.; Pan, J.; Tabatabaei, M.; Aghbashlo, M.; J. Cleaner Prod. 2023, 413, 137473. [Crossref] 61. Sunkar, B.; Bhukya, B.; Frontiers in Energy Research 2022, 10, 802522. [Crossref] 62. Chaihad, N.; Anniwaer, A.; Az Zahra, A. C.; Kasai, Y.; Reubroycharoen, P.; Kusakabe, K.; Abudula, A.; Guan, G.; Bioresour. Technol. 2021, 341, 125874. [Crossref] 63. Mishra, A.; Nanda, S.; Parida, M. R.; Jena, P. K.; Dwibedi, S. K.; Samantaray, S. M.; Samantaray, D.; Mohanty, M. K.; Dash, M.; Bioresour. Technol. 2023, 367, 128231. [Crossref] 64. Narnaware, S. L.; Panwar, N. L.; Bioresour. Technol. Rep. 2022, 17, 100942. [Crossref] 65. Jagnade, P.; Panwar, N. L.; Agarwal, C.; Bioenergy Res. 2023, 16, 1143. [Crossref] 66. Zhong, D.; Zeng, K.; Li, J.; Qiu, Y.; Flamant, G.; Nzihou, A.; Vladimirovich, V. S.; Yang, H.; Chen, H.; Renewable Sustainable Energy Rev. 2022, 157, 111989. [Crossref] 67. Babatabar, M. A.; Yousefian, F.; Mousavi, M. V.; Hosseini, M.; Tavasoli, A.; Int. J. Energy Res. 2022, 46, 9836. [Crossref] 68. Gollakota, A. R. K.; Shu, C. M.; Sarangi, P. K.; Shadangi, K. P.; Rakshit, S.; Kennedy, J. F.; Gupta, V. K.; Sharma, M.; Renewable Sustainable Energy Rev. 2023, 187, 113700. [Crossref] 69. Li, G.; Chen, Y.; Zhang, Z.; Li, B.; Chen, T.; Tian, S.; Postharvest Biol. Technol. 2022, 193, 112057. [Crossref] 70. Ramarao, K. D. R.; Razali, Z.; Somasundram, C.; Kunasekaran, W.; Jin, T. L.; Molecules 2024, 29, 1762. [Crossref] 71. Guedes, P. H. P. S.; Luz, R. F.; Cavalcante, R. M.; Young, A. F.; Biomass Bioenergy 2023, 168, 106659. [Crossref] 72. López-Renau, L. M.; García-Pina, L.; Hernando, H.; Gómez-Pozuelo, G.; Botas, J. A.; Serrano, D. P.; Biomass Convers. Biorefin. 2021, 11, 2311. [Crossref] 73. Dada, T. K.; Sheehan, M.; Murugavelh, S.; Antunes, E.; Biomass Convers. Biorefin. 2021, 13, 2595. [Crossref] 74. Zhang, G.; Zhang, Y.; Zheng, K.; Wang, Q.; Hu, J.; Ind. Crops Prod. 2022, 187, 115518. [Crossref] 75. Tomishige, K.; Yabushita, M.; Cao, J.; Nakagawa, Y.; Green Chem. 2022, 24, 5652. [Crossref] 76. Huang, X.; Ren, J.; Ran, J. Y.; Qin, C. L.; Yang, Z. Q.; Cao, J. P.; Fuel Process. Technol. 2022, 229, 107175. [Crossref] 77. Huonnic, K.; Linclau, B.; Chem. Rev. 2022, 122, 15503. [Crossref] 78. Rover, M. R.; Aui, A.; Wright, M. M.; Smith, R. G.; Brown, R. C.; Green Chem. 2019, 21, 5980. [Crossref] 79. Hakeem, I. G.; Halder, P.; Marzbali, M. H.; Patel, S.; Kundu, S.; Paz-Ferreiro, J.; Surapaneni, A.; Shah, K.; J. Environ. Chem. Eng. 2021, 9, 105614. [Crossref] 80. Ke, L.; Wu, Q.; Zhou, N.; Xiong, J.; Yang, Q.; Zhang, L.; Wang, Y.; Dai, L.; Zou, R.; Liu, Y.; Ruan, R.; Wang, Y.; Renewable Sustainable Energy Rev. 2022, 165, 112607. [Crossref] 81. Zhang, C.; Chao, L.; Zhang, Z.; Zhang, L.; Li, Q.; Fan, H.; Zhang, S.; Liu, Q.; Qiao, Y.; Tian, Y.; Wang, Y.; Hu, X.; Renewable Sustainable Energy Rev. 2021, 135, 110416. [Crossref] 82. Liu, Y.; Cruz-Morales, P.; Zargar, A.; Belcher, M. S.; Pang, B.; Englund, E.; Dan, Q.; Yin, K.; Keasling, J. D.; Cell 2021, 184, 1636. [Crossref] 83. Wei, D.; Chen, C.; Huang, X.; Zhao, J.; Fan, D.; Zeng, T.; Bi, Y.; J. Energy Inst. 2023, 107, 101182. [Crossref] 84. Alcazar-Ruiz, A.; Garcia-Carpintero, R.; Dorado, F.; Sanchez- Silva, L.; Food Bioprod. Process. 2021, 125, 37. [Crossref] 85. Karunanithi, S.; Kapoor, A.; Kumar, P. S.; Balasubramanian, S.; Rangasamy, G.; Sep. Sci. 2023, 58, 1985. [Crossref] 86. Mahfud, F. H.; van Geel, F. P.; Venderbosch, R. H.; Heeres, H. J.; Sep. Sci. 2008, 43, 3056. [Crossref] 87. Alcazar-Ruiz, A.; Villardon, A.; Dorado, F.; Sanchez-Silva, L.; Waste Manage. 2023, 169, 112. [Crossref] 88. Hu, B.; Zhang, Z.; Xie, W.; Liu, J.; Li, Y.; Zhang, W.; Fu, H.; Lu, Q.; Fuel Process. Technol. 2022, 237, 107465. [Crossref] 89. Yu, J.; Wang, D.; Sun, L.; Fuel 2021, 290, 120078.[Crossref] 90. Sangthong, S.; Phetwarotai, W.; Bakar, M. S. A.; Cheirsilp, B.; Phusunti, N.; Ind. Crops Prod. 2022, 188, 115648. [Crossref] 91. Albuquerque, B. R.; Heleno, S. A.; Oliveira, M. B. P. P.; Barros, L.; Ferreira, I. C. F. R.; Food Funct. 2021, 12, 14. [Crossref] 92. Ma, S.; Li, H.; Zhang, G.; Iqbal, T.; Li, K.; Lu, Q.; Front. Environ. Sci. Eng. 2021, 15, 25. [Crossref] 93. Ouedraogo, A. S.; Bhoi, P. R.; Gerdmann, C.; Patil, V.; Adhikari, S.; J. Energy Inst. 2021, 99, 9. [Crossref] 94. Usino, D. O.; Ylitervo, P.; Moreno, A.; Sipponen, M. H.; Richards, T.;J. Anal. Appl. Pyrolysis 2021, 159, 105297. [Crossref] 95. Xue, X.; Mi, J.; Huang, F.; Wang, J.; Chen, L.; Liang, J.; J. Anal. Appl. Pyrolysis 2023, 170, 105909. [Crossref] 96. Zhang, J.; Li, C.; Yuan, H.; Chen, Y.; Renewable Energy 2022, 184, 280. [Crossref]
Associate Editor handled this article: Boniek G. Vaz |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access