Artigo
| Simple and green production of lignin nanoparticles using ethanol-water mixtures and ultrasonication |
|
Eduardo G. F. de AlmeidaI; Lucas G. P. TienneII; Braulio S. ArchanjoIII; Felipe S. AlencastroIV I Programa de Engenharia da Nanotecnologia, Universidade Federal do Rio de Janeiro, 21941-972 Rio de Janeiro - RJ, Brasil Received: 04/20/2025; *e-mail: renata@metalmat.ufrj.br Guest Editor handled this article: Suzana B. Peripolli Lignin nanoparticles (LNPs) have emerged as promising bio-based materials for sustainable applications, but conventional synthesis methods often rely on surfactants, chemical modifications, or toxic solvents. In this study, we present a simple and environmentally friendly approach for producing LNPs using ethanol-water mixtures and ultrasonication, without the use of additives or hazardous reagents. Two solvent compositions (70 and 90% ethanol, v/v in water) and varying sonication times (0-2 h) were evaluated to investigate their effects on nanoparticle formation, morphology, and size. Visual assessment and scanning electron microscopy (SEM) revealed that 70% ethanol promotes spontaneous nanoparticle formation with spherical morphology and good dispersion, even without sonication. In contrast, 90% ethanol required prolonged ultrasonication to produce smaller and more uniform particles. Particle size analysis confirmed that ultrasonication enhances particle refinement, especially in the solvent systems with higher ethanol content. This minimalist, surfactant-free method demonstrates the potential for scalable and green production of lignin nanoparticles, offering a valuable platform for future development of functional bio-based nanomaterials. INTRODUCTION Lignin, the second most abundant biopolymer on Earth, is a complex aromatic macromolecule derived primarily as a by-product from pulping processes. Although traditionally viewed as industrial waste, lignin possesses unique structural features, including phenolic and aliphatic hydroxyl groups, that have attracted increasing attention for value-added applications. Among these, lignin nanoparticles (LNPs) have shown great potential due to their antioxidant, antimicrobial, ultraviolet (UV)-blocking, and biodegradable properties.1 These characteristics position LNPs as promising candidates in pharmaceutical, cosmetic, food packaging, and agricultural fields, particularly under the growing demand for sustainable and environmentally benign materials. Among the various techniques available for lignin nanosizing, solvent shifting, also known as antisolvent precipitation or nanoprecipitation, stands out due to its simplicity, scalability, and compatibility with green chemistry principles. Nanoprecipitation processes typically involve the dissolution of lignin in an organic solvent, followed by its rapid mixing with a non-solvent (antisolvent), usually water, inducing supersaturation and self-assembly of lignin macromolecules into nanostructures.2 The formation of nanoparticles occurs through the aggregation of hydrophobic domains, stabilized by the hydrophilic functional groups on the lignin backbone, forming core-shell-like structures.3 However, conventional solvents used in this technique (e.g., tetrahydrofuran (THF), dimethyl sulfoxide (DMSO)), pose significant environmental and toxicological concerns. Furthermore, complete removal of these solvents often requires time-consuming processes such as dialysis, contradicting the principles of green chemistry. In this sense, ethanol can emerge as a safer and greener alternative due to its low toxicity, renewability, and miscibility with water, and possible use as green solvent to lignin.4 Recent studies5,6 have shown that the use of acetone-water mixtures enables tunable nanoparticle formation, where the solvent polarity can be adjusted to control lignin solubility and nucleation rate. Ethanol-water 70/30% was a method for lignin precipitation used by Figueiredo et al.7 and the results were not promising. Well-formed lignin round particles were obtained preferred by acetone/water solutions. Ultrasonication has also been increasingly employed as an auxiliary method to enhance nanoparticle formation.8-10 The high-energy cavitation effect produced by ultrasound waves promotes more efficient dispersion, breaking down lignin aggregates and accelerating nucleation kinetics without the need for chemical surfactants or stabilizers.11 When combined with ethanol-water systems, ultrasonication can significantly reduce particle size and polydispersity, while improving colloidal stability, which is an important aspect for practical applications.12 A crucial factor in determining the applicability of LNPs lies in their morphology. The size, shape, and surface characteristics of nanoparticles greatly influence their behavior in biological systems, interaction with other materials, and physical stability over time. Scanning electron microscopy (SEM) and atomic force microscopy (AFM) are commonly used techniques to characterize the morphology of lignin nanoparticles. Studies13 have reported a range of morphological outcomes - from spherical to hollow, porous, or even aggregated structures - depending on the lignin source, molecular weight, solvent composition, and processing conditions. For example, spherical and smooth-surfaced nanoparticles are preferred for drug delivery due to their predictable release profiles and lower aggregation tendencies. In contrast, porous or irregularly shaped particles might be advantageous in catalysis or controlled-release fertilizers, where higher surface area is desired.1 In this work, we present a fully green method for the preparation of lignin nanoparticles using ethanol-water mixtures and ultrasonication, without toxic solvents or surfactants. We systematically investigate the effects of solvent ratio and ultrasound treatment time on the morphology, size, and stability of the resulting nanoparticles. The difference between the present work and the papers cited above is that in our case, we first stabilized the mixture of solvents and then introduced the lignin on the already structured solution. Our findings contribute to a deeper understanding of the process-structure relationships in lignin nanoparticle formation, offering insights into their optimization for eco-friendly nanomaterial applications.
EXPERIMENTAL Materials Kraft lignin (alkali, low sulfonated), obtained from Sigma-Aldrich, was used without further purification. Ethanol (≥ 99.5%, analytical grade) was purchased from Vetec and used as received. Ultrapure water (resistivity 18.2 MΩ cm) was obtained using a Milli-Q purification system. All reagents were handled under ambient laboratory conditions. Preparation of lignin nanoparticles via solvent exchange LNPs were synthesized using an environmentally friendly nanoprecipitation approach involving ethanol-water solutions at two different volume ratios 70:30 and 90:10 (ethanol:water, v/v), to investigate the influence of solvent polarity and structuring on nanoparticle formation. In each experiment, 20 mg of lignin were added to 20 mL of ethanol-water solution resulting in a final concentration of 1 mg mL-1. For all formulations, the solvent mixture was prepared prior to lignin addition, and the solid lignin powder was directly introduced into the solution. The dispersions were magnetically stirred for 20 min to promote dissolution and partial solubilization of lignin aggregates. No surfactants, pH adjustments, or chemical additives were used throughout the process, distinguishing this procedure from more complex or chemically-intensive methods reported previously. The final samples were let to decant for 24 h and then poured in a silicon wafer for SEM analysis. Ultrasonication was employed to assist nanoparticle control and reduce particle size. Samples were subjected to ultrasonic treatment using a sonicator bath Quimis 03/350 operating at 40 kHz and 160 W. Sonication was performed for up to 2 h under ambient temperature, while control samples were maintained under magnetic stirring alone (0 h ultrasonication). All dispersions were subsequently stored in falcon tubes for further analysis. Characterization of LNP dispersions Visual analysis and stability observation Immediately after preparation, all LNP suspensions were visually inspected to evaluate clarity, ranging from transparent to yellowish/brownish appearance, and initial sedimentation behavior. The vials were left undisturbed at room temperature, and periodic observations were recorded to monitor the onset of sedimentation or aggregation over time. Photographic documentation was obtained before and after homogenization to compare redispersibility and stability, with visual indicators of particle sedimentation used as a practical measure of colloidal behavior. Photographic documentation was performed 30 days after sample preparation using 5000 K fluorescent lighting and automatic white balance settings to ensure consistent visual conditions. Scanning electron microscopy (SEM) Morphological analysis of the lignin nanoparticles was carried out using scanning electron microscopy. A 1 cm2 piece of silicon wafer was cleaned with isopropyl alcohol, and 10 µL of the nanoparticle suspension were pipetted onto the surface and allowed to dry overnight at room temperature. SEM images were acquired using a field emission SEM system Magellan 400-FEI, operating at an accelerating voltage of 5-10 kV. Morphological differences between particles formed in 70 and 90% ethanol systems, with and without ultrasonication, were analyzed in terms of size, shape uniformity, and degree of aggregation. Fourier transform infrared spectroscopy (FTIR) Infrared spectra were performed on a Shimadzu IR-Tracer 100 Fourier transform infrared spectrometer using direct transmission. The spectra were collected in the range of 4000 to 400 cm-1 with 45 scans and 4 cm-1 resolution. Three spectra were collected for each sample, and the average spectrum was used for analysis. Characterization of particle size analysis via image processing Particle size distribution was evaluated by processing SEM images using ImagePro Plus 6.0 (Media Cybernetics, Rockville, USA), although the approach is applicable to similar image analysis software. Calibration was performed based on the scale bar embedded in each image. Each complete particle was manually counted, with measurements taken twice per particle along perpendicular diameters to ensure accuracy. In cases where particles were conjoined, individual particles were distinguished and counted separately. Manual correction was applied when particle boundaries were ambiguous due to aggregation, charging effects, or shadowing in the micrographs. The collected diameter measurements were then used to calculate the mean particle size and standard deviation for each experimental condition. While this method requires more effort than dynamic light scattering (DLS), it provides greater accuracy for irregularly shaped particles and enables more reliable comparisons between samples.
RESULTS AND DISCUSSION Visual stability and initial observations Figure 1 shows the visual aspect of the lignin dispersions prepared with different ethanol-water ratios and ultrasonication times. Samples ranged from 0 to 2 h of ultrasonic treatment in 70% ethanol, with additional controls using 100% water and 100% ethanol at 0 h. The sample prepared in pure water presented clear sedimentation and poor solubilization, consistent with the low aqueous solubility of lignin. In contrast, samples with 70% ethanol appeared well-dispersed, even without ultrasonication, indicating that this solvent ratio promotes spontaneous nanoparticle formation. The 90% ethanol sample also showed dispersion but with visible coloration differences, suggesting partial solubilization or aggregation behavior. The image was acquired 30 days after sample preparation, under 5000 K fluorescent illumination with automatic white balance.
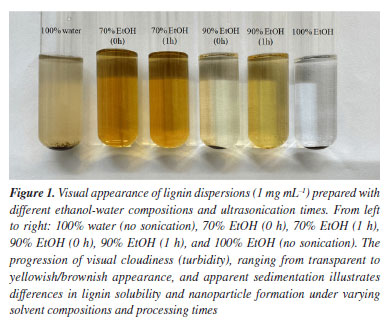
With increasing sonication time (1-2 h), samples in 70% ethanol maintained visual homogeneity and stability, indicating that ultrasonication assists in particle refinement without destabilizing the suspension. In 90% ethanol, dispersions initially formed with some sediment at the bottom, which could be redispersed upon mild shaking, pointing to moderate colloidal stability. These observations align with previous studies5,7 showing that solvent polarity plays a critical role in the solubility and self-assembly behavior of lignin macromolecules. Ethanol-water mixtures, particularly in intermediate proportions such as 70%, provide an optimal polarity range that enhances lignin dispersion and promotes nanoparticle formation via controlled supersaturation during solvent exchange. The better visual stability and homogeneity of 70% ethanol samples, even at 0 h, suggest that this solvent system is effective in promoting spontaneous assembly of lignin into colloidally stable structures. The 90% ethanol system, while also enabling particle formation, showed signs of lower solubility and sedimentation, likely due to reduced hydrogen bonding capacity and increased hydrophobicity. In general, lignin exhibits low solubility in polar solvents like water14 or in nonpolar solvents like hexane.15 Its cross-linked structure prevents it from dispersing easily, as the cross-links and the presence of hydrophobic groups hinder interaction with solvents of distinct polarities. A mixture of ethanol and water is effective in dissolving the lignin due to its intermediate solvent properties.14 Ethanol is an alcohol with both polar and nonpolar characteristics, allowing it to interact with different regions of lignin. Water, in turn, is highly polar and can stabilize the hydrophilic groups present in lignin.16 This combination can break the cross-links, and hydrophobic interactions present in lignin, facilitating its dissolution. Additionally, ethanol can solubilize the aromatic and hydrophobic parts,17 while water helps stabilize the hydrophilic regions,18 creating a favorable environment for dissolution. The mechanism involves the interaction of ethanol with the hydrophobic groups of lignin, penetrating its cross-linked structure, while water stabilizes the hydrophilic regions through hydrogen bonding. This combination reduces the energy interaction between the units of lignin, promoting its dispersion and dissolution. The fact that redispersion could be achieved with minimal agitation further supports the notion that these systems form kinetically trapped but reversible aggregates. Overall, these macroscopic observations provide preliminary yet strong evidence of solvent-driven control over lignin nanoparticle formation and stability. Morphological characterization by SEM and FTIR Representative SEM images (Figure 2) reveal the morphology of LNPs synthesized under selected conditions, specifically, 0, 1, and 2 h of ultrasonication in 70 and 90% ethanol (v/v) mixtures. For LNPs produced in 70% ethanol, the morphology was predominantly spherical with relatively smooth surfaces. At 0 h, particles appeared as larger and slightly polydisperse aggregates, while sonication for 1 and 2 h led to a clear reduction in size and a more homogeneous, monodisperse population. This suggests that ultrasonication facilitates disaggregation and fragmentation of lignin clusters, promoting the formation of uniform nanostructures in moderately polar media.10
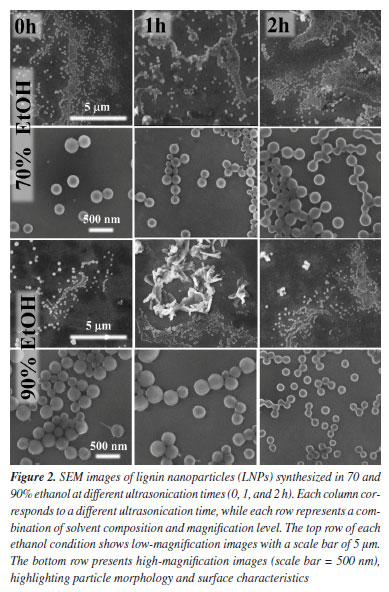
In contrast, the LNPs formed in 90% ethanol displayed greater morphological heterogeneity. After 2 h of sonication, the particles appeared smaller and more defined; however, they remained more irregular than those from the 70% ethanol system. This behavior may stem from the reduced polarity and hydrogen bonding capacity of 90% ethanol, which hinders full solubilization of lignin and facilitates uncontrolled precipitation into irregular structures. These morphological differences are consistent with reports in the literature highlighting the importance of solvent polarity and supersaturation in the nanoprecipitation process. Intermediate ethanol-water mixtures, such as 60-80%, have been shown to provide optimal conditions for lignin self-assembly into spherical nanoparticles due to a balanced rate of nucleation and growth.5,7 The presence of water helps maintain a degree of hydrogen bonding with hydrophilic groups in lignin, while ethanol modulates the hydrophobic interactions required for aggregation. In high ethanol content (90% or above), the reduced dielectric constant and solvation capability can lead to abrupt supersaturation and rapid precipitation, often resulting in less controlled particle morphologies. Moreover, the observed improvements in particle regularity and surface smoothness with increasing ultrasonication time align with previous studies12 demonstrating that ultrasonic energy not only promotes dispersion but can also induce cavitation effects that facilitate the breakup of larger lignin domains into nanoscale units. However, excessive sonication may also cause localized heating or secondary aggregation, which should be considered in future optimization studies. Overall, SEM analysis confirms that both solvent composition and ultrasonic processing play significant roles in determining the morphology of LNPs. The 70% ethanol system appears particularly favorable for producing well-defined, spherical, and uniform nanoparticles with minimal processing steps and no chemical modification, what is an important aspect for scalable green synthesis. The FTIR spectra of the samples are shown in Figure 3. In the spectral region from 400 to 1400 cm-1, no significant chemical differences were observed, aside from minor fluctuations; the sole exception was a slight increase in the intensity of the band at 870 cm-1 in samples prepared with 90% ethanol, which is attributed to aromatic C-H out-of-plane deformations.19 In the higher wavenumber range, a differentiated behavior was noted: the band at 1460 cm-1, corresponding to CH2 and CH3 bending vibrations in aliphatic side chains, exhibited stronger absorption in samples prepared with 70% ethanol, whereas the band near 1600 cm-1, associated with the C=C stretching of aromatic rings,20 was more intense in the 90% EtOH samples.
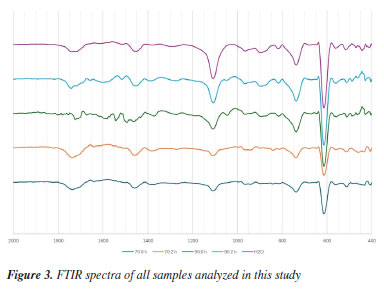
Additionally, absorption at 1730 cm-1, assigned to carbonyl (C=O) stretching,21 was more pronounced in the 70% EtOH group. These findings suggest a preferential expression of aliphatic functional groups in samples treated with lower ethanol content, while higher ethanol concentrations favored the spectral features associated with aromatic moieties. Since the treatment did not produce any new peaks, nor did any existing peak disappear, these observations support the interpretation that no chemical transformations occurred during the treatment. Therefore, the observed spectral variations are consistent with conformational rearrangements in the lignin macromolecular structure via π-π interaction, rather than chemical modifications. Particle size analysis The quantitative analysis of nanoparticle size based on SEM image processing using ImagePro Plus 6.0 is presented in Figure 4 and summarized in Table 1. The data show a clear trend of decreasing average particle diameter with increasing ultrasonication time, particularly in the 70% ethanol system. For example, nanoparticles produced in 70% ethanol without ultrasonication exhibited an average diameter of 256 nm, which progressively decreased to 149 nm after 2 h of ultrasonication. In contrast, samples prepared in 100% ethanol under similar conditions showed smaller size variations, with average diameters ranging from 162 to 140 nm. These results confirm that both solvent composition and sonication duration significantly influence nanoparticle size, with aqueous ethanol mixtures exhibiting greater responsiveness to mechanical energy input. The observed trends are consistent with the morphological features described earlier and underscore the role of interfacial dynamics and energy input in governing nanoprecipitation outcomes.
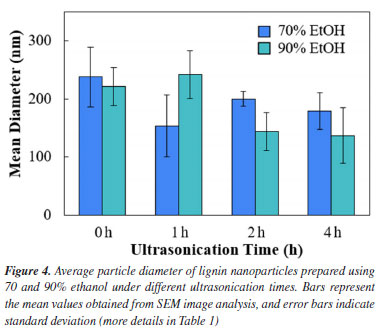
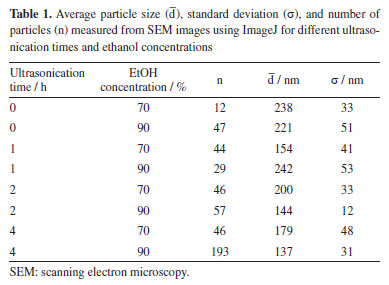
A general trend of particle size reduction was observed with increasing sonication time, particularly in the 90% ethanol group. For 70% ethanol, particles initially decreased in size after 1 h of ultrasonication, reaching an average of 154 nm, but then increased slightly for the 2 h mark, and remained relatively stable for a time of 4 h. This behavior may suggest a threshold in the fragmentation capacity of ultrasonication, where further energy input does not significantly alter the particle size due to stabilization of the nanoparticle population. Such a plateau has also been observed in previous studies,22 where prolonged ultrasonication led to no significant further reduction in particle size, likely due to equilibrium between dispersion and reaggregation forces. In contrast, the particle size in 90% ethanol increased from 221 nm (0 h) to 242 nm (1 h), dropping to 144 nm at the 2 h mark and further to 137 nm after another 2 h. This trend suggests that ultrasonication is more efficient in reducing particle size when lignin is initially less solubilized, which is possibly due to the presence of larger, loosely associated lignin domains that are more susceptible to mechanical disruption. The high ethanol content may enhance cavitation intensity and localized shear forces, which promote the breakdown of aggregates, despite the lower spontaneous nanoprecipitation efficiency. This is consistent with previous reports12,23 where ultrasonication was shown to improve nanoparticle uniformity and reduce polydispersity, particularly in systems with limited initial solubility. The broader particle size distribution and higher standard deviations observed in some conditions may reflect transient instability or ongoing aggregation-disaggregation dynamics. Our findings highlight the need to fine-tune sonication parameters for effective size reduction and also to ensure reproducibility and stability of the resulting nanoparticle suspensions. The 70% ethanol system provides a favorable environment for spontaneous and stable nanoparticle formation, while 90% ethanol benefits more from prolonged ultrasonication to achieve smaller particle sizes. This highlights the interplay between solvent-induced self-assembly and mechanical dispersion in nanoparticle engineering. Beyond the morphological and dimensional features, the visual and SEM-based observations suggest distinct particle formation mechanisms between 70 and 90% ethanol systems. In the 70% ethanol medium, the relatively high polarity and hydrogen bonding capacity of water favor gradual supersaturation and controlled nucleation of lignin domains, facilitating the spontaneous assembly into spherical nanoparticles.5,7 In contrast, the higher ethanol concentration in the 90% system may lead to more abrupt precipitation events due to limited lignin solubilization, resulting in broader particle size distributions and less defined shapes. These differences are indicative of the competing roles of solvation, diffusion, and interfacial tension in dictating nanoprecipitation dynamics, an aspect that could be further clarified by time-resolved studies or in situ analysis techniques. Another relevant aspect to consider is the behavior of ethanol-water mixtures, which are known to form transient micro-heterogeneous structures or "clusters" due to preferential hydrogen bonding. These clusters typically exhibit a hydrophobic interior and a hydrophilic shell,24 potentially influencing the nucleation and assembly of lignin into nanoparticles. In this context, the 70:30 ethanol-water ratio may represent a composition where such clusters are most stable and abundant, offering a soft template that promotes the self-organization of lignin macromolecules into well-defined nanoparticles. The preparation method used in this study, where the lignin was introduced into a pre-equilibrated ethanol-water solution, may have favored alignment along these microstructural domains, facilitating uniform aggregation and enhancing colloidal stability. This cluster-templated mechanism could help explain the favorable nanoparticle formation observed at 70% ethanol, even without ultrasonication. The simplicity of the formulation, using only ethanol, water, and lignin, did not compromise the quality or stability of the resulting dispersions. This emphasizes the potential of ethanol-water systems as viable platforms for the scalable and sustainable production of functional nanoparticles.12 The results suggest that careful tuning of ethanol content and ultrasonication time can be used as effective levers to tailor LNP size and uniformity, without the need for pH control, additives, or complex post-processing.23 These advantages not only contribute to the green chemistry profile of the method but also make it attractive for industrial applications where simplicity and reproducibility are essential. Also, it is worth noting that additional characterization techniques, such as dynamic light scattering (DLS), zeta potential measurements, Fourier transform infrared spectroscopy (FTIR), and ultraviolet-visible (UV-Vis) spectroscopy, can provide deeper insight into the physicochemical properties and colloidal stability of lignin nanoparticles. While these analyses were beyond the scope of the present study, they are currently being explored by our group to expand upon the current findings and offer a more comprehensive understanding of the self-assembly mechanisms and potential applications of these bio-based nanomaterials.
CONCLUSIONS In this study, we demonstrated a simple and eco-friendly method for producing LNPs using only ethanol-water mixtures and ultrasonication, without the need for surfactants, toxic solvents, or chemical modifications. The results highlight the influence of both solvent composition and sonication time on nanoparticle formation, size, and morphology. Particularly, 70% ethanol promoted spontaneous nanoparticle formation with good stability and spherical morphology, while 90% ethanol required longer sonication to achieve similar results, suggesting different nanoprecipitation mechanisms governed by solvent polarity. The current findings provide additional understanding of how solvent polarity and sonication conditions influence lignin nanoparticle formation using a minimalistic green approach. By avoiding toxic solvents, surfactants, or chemical modifications, the methodology simplifies the synthesis process and also aligns with circular economy and green chemistry principles. Moreover, this simplified system serves as a platform for more in-depth studies, including kinetic modeling of nanoparticle growth, surface chemistry analysis, and long-term performance evaluation. The ability to achieve tunable particle sizes and stable colloidal suspensions using only ethanol and water positions this method as a viable alternative for the sustainable production of bio-based nanomaterials. Future studies by our group are expanding upon these results by investigating a broader range of solvent ratios, lignin concentrations, and ultrasonication conditions, as well as employing more advanced characterization techniques to deepen the understanding of nanoparticle properties and functionality. Together, these efforts aim to support the development of lignin-based nanomaterials for applications in packaging, biomedicine, and other fields demanding sustainable and scalable nanotechnologies.
DATA AVAILABILITY STATEMENT The data that support the findings of this study are available from the corresponding author upon request.
REFERENCES 1. Iravani, S.; Varma, R. S.; Green Chem. 2020, 22, 612. [Crossref] 2. Girard, V.; Marshall-Heussler, L.; Chapuis, H.; Brosse, B.; Canilho, N.; Ziegler-Devin, I.; Nanomaterials 2024, 14, 1786. [Crossref] 3. Wang, J.; Qian, Y.; Li, L.; Qiu, X.; ChemSusChem 2020, 13, 4420. [Crossref] 4. Jasiukaitytė-Grojzdek, E.; Kozmelj, T. R.; Tofani, G.; Segers, B.; Nimmegeers, P.; Billen, P.; Pogorevc, R.; Likozar, B.; Grilc, M.; ACS Sustainable Chem. Eng. 2025, 13, 3452. [Crossref] 5. Kim, G.; Park, J.; Kim, B. M.; Kim, J.; Kim, K. J.; Park, J.; Colloids Surf., A 2024, 682, 132803. [Crossref] 6. Li, S.; Zhou, S.; Zhao, G.; Ind. Crops Prod. 2021, 171, 113860. [Crossref] 7. Figueiredo, P.; Lahtinen, M. H.; Agustin, M. B.; Carvalho, D. M.; Hirvonen, S. P.; Penttilä, P. A.; Mikkonen, K. S.; ChemSusChem 2021, 14, 4718. [Crossref] 8. Oliveira, C. J. F.; Freitas, S. K. S.; Sousa, I. G. P. P.; Esteves, P. M.; Simao, R. A.; Colloids Surf., A 2020, 585, 124086. [Crossref] 9. Rao, P.; Geckeler, K. E.; Prog. Polym. Sci. 2011, 36, 887. [Crossref] 10. Ruiz, E.; Orozco, V. H.; Hoyos, L. M.; Giraldo, L. F.; Eur. Polym. J. 2022, 173, 111307. [Crossref] 11. Gilca, I. A.; Popa, V. I.; Crestini, C.; Ultrason. Sonochem. 2015, 23, 369. [Crossref] 12. Ago, M.; Huan, S.; Borghei, M.; Raula, J.; Kauppinen, E. I.; Rojas, O. J.; ACS Sustainable Chem. Eng. 2016, 8, 23302. [Crossref] 13. Schneider, W. D. H.; Dillon, A. J. P.; Camassola, M.; Biotechnol. Adv. 2021, 47, 107685. [Crossref] 14. Melro, E.; Alves, L.; Antunes, F. E.; Medronho, B.; J. Mol. Liq. 2018, 265, 578. [Crossref] 15. Ponnuchamy, V.; Gordobil, O.; Diaz, R. H.; Sandak, A.; Sandak, J.; Int. J. Biol. Macromol. 2021, 168, 792. [Crossref] 16. Sharp, K. A.; Vanderkooi, J. M.; Acc. Chem. Res. 2009, 43, 231. [Crossref] 17. Qu, Y.; Harte, F. M.; Elias, R. J.; Coupland, J. N.; Food Hydrocolloids 2018, 77, 454. [Crossref] 18. Xu, F.; Wei, M.; Zhang, X.; Song, Y.; Zhou, W.; Wang, Y.; Research 2019, 2019, 2581241. [Crossref] 19. Salim, R. M.; Asik, J.; Sarjadi, M. S.; Wood Sci. Technol. 2021, 55, 295. [Crossref] 20. Feng, S.; Yuan, Z.; Leitch, M.; Xu, C. C.; Fuel 2014, 116, 214. [Crossref] 21. Zhang, M.; Thomas, N. L.; Appl. Polym. Sci. 2009, 116, 688. [Crossref] 22. Wan, Z.; Zhang, H.; Niu, M.; Zhang, W.; Guo, Y.; Li, H.; Ind. Crops Prod. 2025, 224, 120394. [Crossref] 23. Liu, X.; Xie, M.; Hu, Y.; Li, S.; Nie, S.; Zhang, A.; Wu, H.; Li, C.; Xiao, Z.; Hu, C.; Ind. Crops Prod. 2022, 183, 114943. [Crossref] 24. Dixit, S.; Crain, J.; Poon, W. C. K.; Finney, J. L.; Soper, A. K.; Nature 2002, 416, 829. [Crossref] |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access






